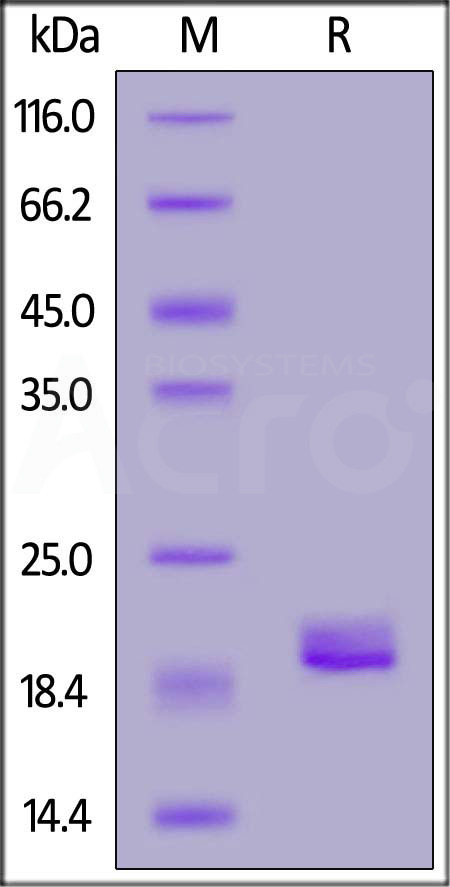
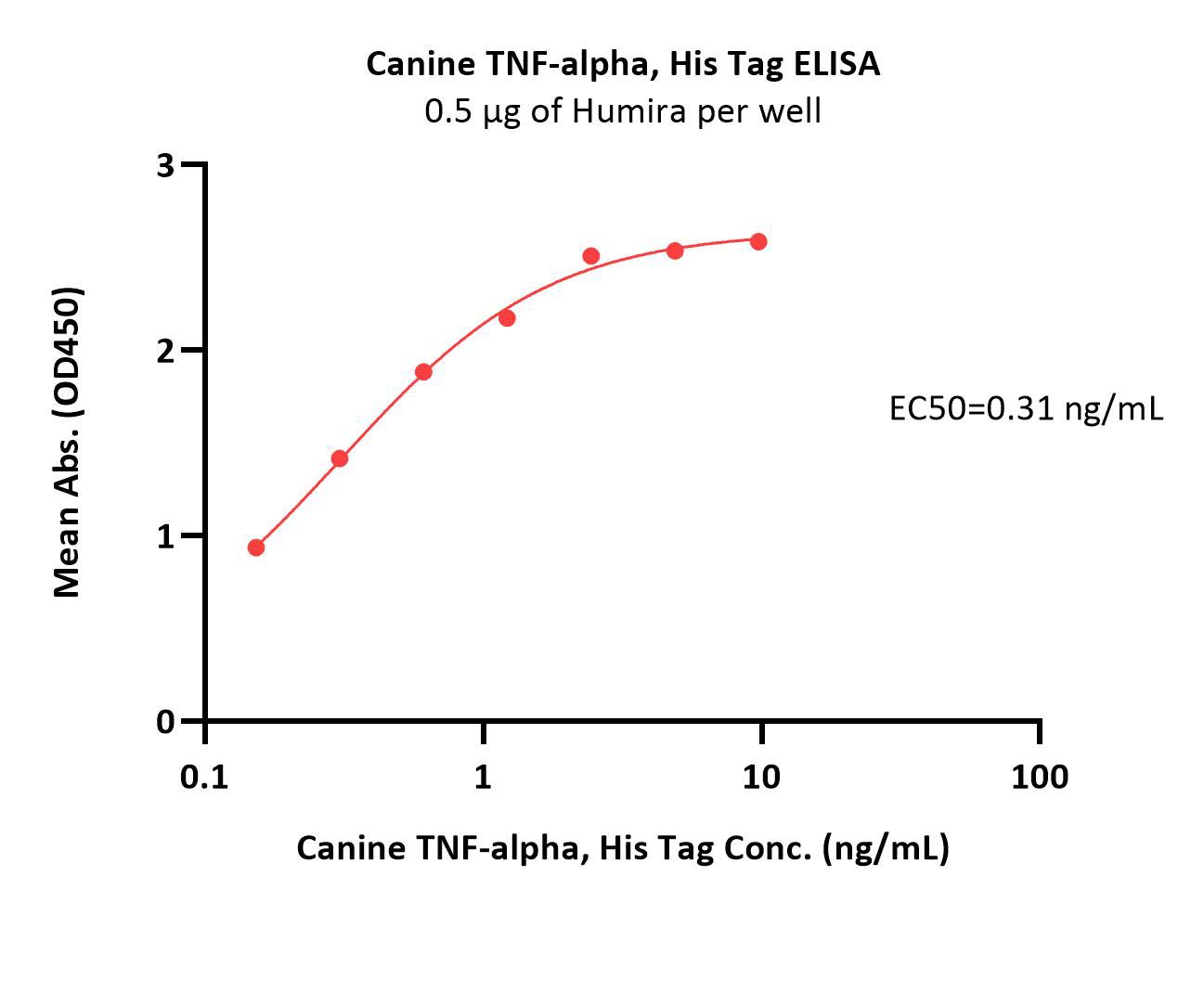
| Etanercept biosimilar (Hanwha Chemical) |
HD-203; MK-8953 |
Approved |
Hanwha Chemical |
Davictrel |
|
|
|
|
Spondylitis, Ankylosing; Arthritis, Rheumatoid; Psoriasis; Arthritis, Psoriatic |
Details
|
| Anti-tumour necrosis factor-alpha antibody |
|
Approved |
Materia Medica Holding Research And Production Company |
Artrofoon, Arthrofoon |
|
|
|
|
Arthritis, Rheumatoid; Osteoarthritis |
Details
|
| Etanercept biosimilar (AryoGen Biopharma) |
|
Approved |
Aryogen Biopharma |
Altebrel |
|
|
|
|
Spondylitis, Ankylosing; Arthritis, Rheumatoid; Arthritis, Juvenile; Psoriasis; Arthritis, Psoriatic |
Details
|
| Adalimumab biosimilar (Reliance Life Sciences) |
R-TPR-021 |
Approved |
Reliance Life Sciences |
Adfrar IB |
India |
Spondylitis, Ankylosing; Arthritis, Rheumatoid; Colitis, Ulcerative; Psoriasis; Arthritis, Psoriatic |
Torrent Pharma, Reliance Life Sciences |
2016-01-12 |
Spondylitis, Ankylosing; Arthritis, Rheumatoid; Psoriasis; Colitis, Ulcerative; Arthritis, Psoriatic |
Details
|
| Certolizumab pegol |
PHA-738144; CDP-870; CZP |
Approved |
Ucb |
希敏佳, Cimzia, Cimziat |
United States |
Crohn Disease |
Ucb Inc |
2008-04-22 |
Non-radiographic axial spondyloarthritis; Infertility, Male; Polyarticular Juvenile Idiopathic Arthritis; Spondylitis, Ankylosing; Arthritis, Rheumatoid; Spondylarthropathies; Spondylarthritis; Psoriasis; Arthritis, Psoriatic; Cystitis, Interstitial; Colitis, Ulcerative; Osteoarthritis, Spine; Crohn Disease; Plaque psoriasis |
Details
|
| Etanercept biosimilar (Nanogen Biopharmaceutical) |
|
Approved |
Nanogen Biopharmaceutical Co |
Nanercept |
Vietnam |
Arthritis, Rheumatoid; Psoriasis |
Nanogen Biopharmaceutical Co |
2019-01-01 |
Arthritis, Rheumatoid; Psoriasis |
Details
|
| Infliximab Biosimilar (Genor Biopharma) |
GB-242 |
Approved |
Genor Biopharma Co Ltd |
佳佑健 |
Mainland China |
Arthritis, Rheumatoid; Colitis, Ulcerative; Spondylitis, Ankylosing; Crohn Disease; Psoriasis |
Yuxi Genor Biotechnology Co Ltd |
2022-02-23 |
Arthritis, Rheumatoid; Spondylitis, Ankylosing; Psoriasis; Colitis, Ulcerative; Crohn Disease |
Details
|
| Etanercept biosimilar (Mochida) |
LBEC-0101 |
Approved |
Mochida Pharmaceutical Co Ltd |
|
Japan |
Arthritis, Juvenile; Arthritis, Rheumatoid |
null |
2018-01-19 |
Arthritis, Rheumatoid; Arthritis, Juvenile |
Details
|
| Adalimumab biosimilar (Amgen) |
ABP-501 |
Approved |
Amgen Inc |
Amjevita, Amgevita, Solymbic |
United States |
Colitis, Ulcerative; Crohn Disease; Psoriasis; Arthritis, Psoriatic; Spondylitis, Ankylosing; Arthritis, Juvenile; Arthritis, Rheumatoid |
Amgen Inc |
2016-09-23 |
Non-radiographic axial spondyloarthritis; Polyarticular Juvenile Idiopathic Arthritis; Arthritis, Rheumatoid; Pediatric Crohn's disease; Spondylitis, Ankylosing; Arthritis, Juvenile; Pediatric ulcerative colitis; Psoriasis; Arthritis, Psoriatic; Colitis, Ulcerative; Hidradenitis Suppurativa; Uveitis; Plaque psoriasis; Crohn Disease |
Details
|
| Etanercept biosimilar (PROBIOMED) |
|
Approved |
Probiomed |
Infinitam |
Mexico |
Arthritis, Rheumatoid |
Probiomed |
2014-01-01 |
Arthritis, Rheumatoid |
Details
|
| Adalimumab biosimilar (Fresenius Kabi) |
MSB-11022 |
Approved |
Merck Serono, Fresenius Kabi Ag |
Idacio, Kromeya |
EU |
Crohn Disease; Uveitis; Hidradenitis Suppurativa; Psoriasis; Arthritis, Psoriatic |
Fresenius Kabi Deutschland Gmbh |
2019-04-02 |
Non-radiographic axial spondyloarthritis; Arthritis, Rheumatoid; Spondylitis, Ankylosing; Arthritis, Juvenile; Psoriasis; Colitis, Ulcerative; Arthritis, Psoriatic; Uveitis; Hidradenitis Suppurativa; Plaque psoriasis; Crohn Disease |
Details
|
| Etanercept biosimial (Sandoz) |
GP-2015 |
Approved |
Clariant Produkte (Schweiz) Ag |
Erelzi |
United States |
Psoriasis; Arthritis, Rheumatoid; Arthritis, Psoriatic; Spondylitis, Ankylosing; Arthritis, Juvenile |
Sandoz |
2016-08-30 |
Arthritis, Rheumatoid; Spondylitis, Ankylosing; Arthritis, Juvenile; Psoriasis; Arthritis, Psoriatic; Plaque psoriasis |
Details
|
| Adalimumab biosimilar (Sinocelltech) |
SCT-630 |
Approved |
SinoCelltech Ltd |
安佳润 |
Mainland China |
Arthritis, Juvenile; Crohn Disease; Pediatric Crohn's disease; Arthritis, Rheumatoid; Uveitis; Psoriasis; Spondylitis, Ankylosing |
SinoCelltech Ltd |
2023-06-07 |
Pediatric Crohn's disease; Arthritis, Rheumatoid; Spondylitis, Ankylosing; Arthritis, Juvenile; Psoriasis; Uveitis; Crohn Disease; Plaque psoriasis |
Details
|
| Lenalidomide |
IMID-5013; CDC-5013; CDC-501; CC-5013; IMiD-3; ENMD-0997; STAR-LLD |
Approved |
Celgene Corp |
Revimid (former Brand Name), 瑞复美, Revlimid, Leavdo |
United States |
Myelodysplastic Syndromes |
Bristol Myers Squibb Srlcompany |
2005-12-27 |
Lymphoma, T-Cell, Peripheral; Optic Nerve Glioma; Intestinal Neoplasms; Immunoproliferative Small Intestinal Disease; Solid tumours; Bone Marrow Neoplasms; Lymphoma, B-Cell, Marginal Zone; Kidney Neoplasms; Leukemia; Liver Neoplasms; Hematologic Diseases; Leukemia, Erythroblastic, Acute; Leukemia, Promyelocytic, Acute; Leukemia, Myeloid; Ependymoma; HIV Infections; Ovarian Neoplasms; Medulloblastoma; Leukemia, Hairy Cell; Anemia; Paraproteinemias; Pain; Polycythemia Vera; Plaque, Amyloid; Rhabdoid Tumor; Anemia, Refractory, with Excess of Blasts; Glioblastoma; Smoldering Multiple Myeloma; Anemia, Refractory; Lymphoma, Large B-Cell, Diffuse; Hodgkin Disease; Myelodysplastic Syndromes; Hypothalamic Neoplasms; Neoplasms; Leukemia-Lymphoma, Adult T-Cell; Plasmacytoma; Blood Protein Disorders; Graft vs Host Disease; Leukemia, Myelomonocytic, Chronic; Leukemia, Myelomonocytic, Acute; Nerve Degeneration; Lymphomatoid Granulomatosis; Pancreatic Neoplasms; Multiple Myeloma; Leukemia, Megakaryoblastic, Acute; Oligodend |
Details
|
| Etanercept biosimilar (Amega Biotech) |
|
Approved |
Amega Biotech |
Enerceptan |
Argentina |
Arthritis, Rheumatoid |
Amega Biotech |
2012-01-01 |
Arthritis, Rheumatoid |
Details
|
| Infliximab biosimilar (Reliance Life Sciences) |
BOW-015; R-TPR-015 |
Approved |
Epirus Biopharmaceuticals Inc |
Infimab |
|
|
|
|
Arthritis, Rheumatoid |
Details
|
| Adalimumab biosimilar (Celltrion) |
CT-P17 |
Approved |
Celltrion Inc |
Yuflyma |
EU |
Arthritis, Psoriatic; Psoriasis; Arthritis, Juvenile; Plaque psoriasis; Pediatric Crohn's disease; Spondylitis, Ankylosing; Pediatric ulcerative colitis; Crohn Disease; Uveitis; Arthritis, Rheumatoid; Hidradenitis Suppurativa; Colitis, Ulcerative |
Celltrion Healthcare Hungary Kft |
2021-02-11 |
Spondylitis, Ankylosing; Pediatric Crohn's disease; Arthritis, Rheumatoid; Arthritis, Juvenile; Psoriasis; Pediatric ulcerative colitis; Arthritis, Psoriatic; Colitis, Ulcerative; Hidradenitis Suppurativa; Uveitis; Plaque psoriasis; Crohn Disease |
Details
|
| Etanercept biosimilar (Intas Biopharmaceuticals) |
|
Approved |
Intas Biopharmaceuticals |
Intacept |
India |
Arthritis, Psoriatic; Spondylitis, Ankylosing; Arthritis, Juvenile; Arthritis, Rheumatoid; Psoriasis |
Intas Biopharmaceuticals |
2015-03-01 |
Spondylitis, Ankylosing; Arthritis, Rheumatoid; Arthritis, Juvenile; Psoriasis; Arthritis, Psoriatic |
Details
|
| Infliximab biosimilar (Amgen) |
ABP-710 |
Approved |
Amgen Inc |
Avsola |
United States |
Pediatric ulcerative colitis; Arthritis, Psoriatic; Spondylitis, Ankylosing; Arthritis, Rheumatoid; Colitis, Ulcerative; Crohn Disease; Pediatric Crohn's disease; Plaque psoriasis |
Amgen Inc |
2019-12-06 |
Arthritis, Rheumatoid; Spondylitis, Ankylosing; Pediatric Crohn's disease; Pediatric ulcerative colitis; Arthritis, Psoriatic; Colitis, Ulcerative; Crohn Disease; Plaque psoriasis |
Details
|
| Adalimumab biosimilar (LG Life Sciences) |
LBAL |
Approved |
Lg Chemical Ltd, Mochida Pharmaceutical Co Ltd |
|
Japan |
Arthritis, Rheumatoid; Psoriasis; Arthritis, Psoriatic; Arthritis, Juvenile; Colitis, Ulcerative; Spondylitis, Ankylosing; Behcet Syndrome; Uveitis, Intermediate; Uveitis, Posterior; Panuveitis; Crohn Disease |
Mochida Pharmaceutical Co Ltd |
2021-03-23 |
Uveitis, Intermediate; Arthritis, Rheumatoid; Spondylitis, Ankylosing; Arthritis, Juvenile; Psoriasis; Arthritis, Psoriatic; Colitis, Ulcerative; Uveitis, Posterior; Behcet Syndrome; Panuveitis; Crohn Disease |
Details
|
| Adalimumab biosimilar (Boehringer Ingelheim) |
BI-695501 |
Approved |
C.H. Boehringer Sohn Ag & Co. Kg |
Cyltezo |
United States |
Colitis, Ulcerative; Crohn Disease; Arthritis, Psoriatic; Spondylitis, Ankylosing; Arthritis, Juvenile; Arthritis, Rheumatoid; Psoriasis |
Boehringer Ingelheim Gmbh |
2017-08-25 |
Arthritis, Rheumatoid; Spondylitis, Ankylosing; Arthritis, Juvenile; Psoriasis; Colitis, Ulcerative; Arthritis, Psoriatic; Hidradenitis Suppurativa; Uveitis; Crohn Disease |
Details
|
| Golimumab |
CNTO-148; rTNV148B |
Approved |
Johnson & Johnson Innovative Medicine |
Simponi/Simponi aria, Simponi, Simponi aria |
United States |
Spondylitis, Ankylosing; Arthritis, Psoriatic; Arthritis, Rheumatoid |
Centocor Ortho Biotech Inc |
2009-04-24 |
Non-radiographic axial spondyloarthritis; Diabetes Mellitus, Type 1; Arthritis, Rheumatoid; Spondylitis, Ankylosing; Arthritis, Juvenile; Colitis, Ulcerative; Arthritis, Psoriatic; Asthma; Labyrinth Diseases; Crohn Disease |
Details
|
| Adalimumab biosimilar (Mylan/Biocon) |
BMO-2; MYL-1401-A |
Approved |
Biocon Ltd |
|
United States |
Spondylitis, Ankylosing; Arthritis, Juvenile; Arthritis, Rheumatoid; Colitis, Ulcerative; Crohn Disease; Plaque psoriasis; Arthritis, Psoriatic |
Mylan Pharmaceuticals Inc |
2020-07-06 |
Spondylitis, Ankylosing; Arthritis, Rheumatoid; Arthritis, Juvenile; Psoriasis; Colitis, Ulcerative; Arthritis, Psoriatic; Hidradenitis Suppurativa; Crohn Disease; Plaque psoriasis |
Details
|
| Luminol sodium |
MP-1032 |
Approved |
Selvim, Metrio |
|
|
Immune System Diseases; Psoriasis |
null |
1997-01-01 |
Coronavirus Disease 2019 (COVID-19); Immune System Diseases; Psoriasis |
Details
|
| Adalimumab biosimilar (Innovent Biologics) |
IBI-303 |
Approved |
Innovent Biologics(Suzhou) Co Ltd |
苏立信 |
Mainland China |
Spondylitis, Ankylosing; Arthritis, Rheumatoid; Psoriasis |
Innovent Biologics(Suzhou) Co Ltd |
2020-09-02 |
Pediatric Crohn's disease; Spondylitis, Ankylosing; Arthritis, Rheumatoid; Arthritis, Juvenile; Psoriasis; Uveitis; Crohn Disease; Plaque psoriasis |
Details
|
| Infliximab biosimilar (Pfizer) |
PF-6438179; GP-1111; PF-06438179 |
Approved |
Pfizer Inc |
Ixifi, Zessly |
EU |
Pediatric Crohn's disease; Pediatric ulcerative colitis; Spondylitis, Ankylosing; Arthritis, Rheumatoid; Colitis, Ulcerative; Crohn Disease; Psoriasis; Arthritis, Psoriatic |
Pfizer Europe Ma Eeig |
2013-09-09 |
Pediatric Crohn's disease; Spondylitis, Ankylosing; Arthritis, Rheumatoid; Pediatric ulcerative colitis; Psoriasis; Colitis, Ulcerative; Arthritis, Psoriatic; Crohn Disease; Plaque psoriasis |
Details
|
| Adalimumab biosimilar (Bio-Thera Solutions) |
BAT-1406 |
Approved |
Bio-Thera Solutions Ltd |
格乐立 |
Mainland China |
Spondylitis, Ankylosing; Arthritis, Rheumatoid; Crohn Disease; Uveitis; Psoriasis |
Bio-Thera Solutions Ltd |
2019-11-04 |
Non-radiographic axial spondyloarthritis; Spondylitis, Ankylosing; Arthritis, Rheumatoid; Psoriasis; Uveitis; Crohn Disease |
Details
|
| Adalimumab biosimilar (Alvotech) |
AVT-02 (Alvotech) |
Approved |
Alvotech hf |
SIMLANDI, Ardalicip, Ciptunec, HUKYNDRA, LIBMYRIS |
EU |
Uveitis; Hidradenitis Suppurativa; Arthritis, Psoriatic; Spondylitis, Ankylosing; Arthritis, Rheumatoid; Crohn Disease; Psoriasis; Colitis, Ulcerative |
Stada Arzneimittel Ag |
2021-11-12 |
Spondylitis, Ankylosing; Arthritis, Rheumatoid; Arthritis, Juvenile; Colitis; Psoriasis; Colitis, Ulcerative; Arthritis, Psoriatic; Uveitis; Hidradenitis Suppurativa; Inflammation; Crohn Disease; Plaque psoriasis |
Details
|
| Adalimumab biosimilar (Biocad) |
BCD-057 |
Approved |
Biocad |
|
Russian Federation |
Psoriasis |
Biocad |
2019-03-01 |
Psoriasis |
Details
|
| Adalimumab biosimilar (Shanghai Henlius Biotech) |
HLX-03 |
Approved |
Shanghai Henlius Biotech Inc |
汉达远 |
Mainland China |
Arthritis, Rheumatoid; Spondylitis, Ankylosing; Psoriasis |
Shanghai Henlius Biotech Inc |
2020-12-02 |
Uveitis, Intermediate; Arthritis, Rheumatoid; Spondylitis, Ankylosing; Immune System Diseases; Psoriasis; Uveitis, Posterior; Plaque psoriasis; Panuveitis |
Details
|
| Infliximab |
TA-650 |
Approved |
Johnson & Johnson Innovative Medicine |
Remicade, 类克, Avakine, CenTNF |
United States |
Crohn Disease |
Centocor |
1998-08-24 |
Uveitis; Vitreoretinopathy, Proliferative; Diabetic macular oedema; Visual Acuity; Pediatric ulcerative colitis; Glaucoma; Arthritis, Psoriatic; Colitis, Ulcerative; Retinal Detachment; Aneurysm; Parapsoriasis; Psoriasis; Hidradenitis Suppurativa; Granulomatous Disease, Chronic; Pulmonary Disease, Chronic Obstructive; Crohn Disease; Stevens-Johnson Syndrome; Behcet Syndrome; Diabetic Retinopathy; Melanoma; Plaque psoriasis; Pediatric Crohn's disease; Polymyositis; Inflammatory Bowel Diseases; Macular Edema; Scleritis; Dermatomyositis; Giant Cell Arteritis; Pancreatitis; Arthritis, Rheumatoid; Spondylitis, Ankylosing; Graft vs Host Disease; Non-radiographic axial spondyloarthritis; Vasculitis; Bipolar Disorder; Spondylarthropathies; Sarcoidosis; Coronavirus Disease 2019 (COVID-19); Mucocutaneous Lymph Node Syndrome; Polymyalgia Rheumatica; Stroke; Arthritis, Juvenile |
Details
|
| Infliximab biosimilar (Shanghai Biomabs) |
CMAB-008; STI-002 |
Approved |
Shanghai Biomabs Pharmaceuticals Co Ltd |
百类停, 类停 |
Mainland China |
Arthritis, Rheumatoid; Pediatric Crohn's disease; Crohn Disease; Spondylitis, Ankylosing; Colitis, Ulcerative; Psoriasis |
Taizhou Mabtech Pharmaceutical Co Ltd |
2021-07-12 |
Arthritis, Rheumatoid; Pediatric Crohn's disease; Spondylitis, Ankylosing; Psoriasis; Colitis, Ulcerative; Crohn Disease |
Details
|
| Infliximab biosimilar (Hisun Pharma) |
HS-626 |
Approved |
Zhejiang Hisun Pharmaceutical Co Ltd |
|
Mainland China |
Psoriasis |
Hisun Biopharmaceutical Co Ltd |
2021-09-24 |
Psoriasis |
Details
|
| Infliximab biosimilar (Bionovis/The Instituto Vital Brazil) |
|
Approved |
Bionovis, The Instituto Vital Brazil |
|
|
|
|
|
Arthritis, Rheumatoid; Spondylitis, Ankylosing; Psoriasis; Arthritis, Psoriatic; Colitis, Ulcerative; Crohn Disease |
Details
|
| Etanercept biosimilar (Mylan) |
YLB-113 |
Approved |
Lupin Ltd, Mylan Nv |
Nepexto |
EU |
Plaque psoriasis; Arthritis, Juvenile; Arthritis, Rheumatoid; Spondylarthropathies; Spondylitis, Ankylosing; Arthritis, Psoriatic |
Biosimilar Collaborations Ireland Ltd |
2020-05-20 |
Arthritis, Rheumatoid; Spondylarthropathies; Spondylitis, Ankylosing; Arthritis, Juvenile; Arthritis, Psoriatic; Plaque psoriasis |
Details
|
| Pomalidomide |
IMID-4047; CDC-394; CC-4047; IMiD-1 |
Approved |
Celgene Corp |
Pomalyst, Imnovid, Actimid, Pomalyst/Imnovid, 安跃 |
United States |
Multiple Myeloma |
Bristol-Myers Squibb Company |
2013-02-08 |
Pulmonary Fibrosis; Atypical Squamous Cells of the Cervix; Sarcoma, Kaposi; Anemia, Sickle Cell; Neurofibromatosis 1; Lymphoma, Non-Hodgkin; Carcinoma, Small Cell; Waldenstrom Macroglobulinemia; Glioma; Primary Myelofibrosis; Sarcoma; Thrombocytosis; Prostatic Neoplasms; Lung Diseases, Interstitial; Medulloblastoma; Central Nervous System Neoplasms; Multiple Myeloma; Hodgkin Disease; Plasmacytoma; Kidney Diseases; Pancreatic Neoplasms; Immunoglobulin Light-chain Amyloidosis; Myeloproliferative Disorders; Graft vs Host Disease; Scleroderma, Systemic; Polycythemia Vera; Bone Marrow Neoplasms; Solid tumours |
Details
|
| Adalimumab biosimilar (Jiangsu Zhonghe/Shanghai Junshi/Jiangsu T-Mab) |
9MW-0113; 9MW0113; UBP-1211; 9-MW-0113; 9-MW0113 |
Approved |
Shanghai Junshi Biosciences Co Ltd, Jiangsu Zhonghe Pharmaceutical Technology Co Ltd, Jiangsu T-Mab Bio-Pharmaceuticals Co Ltd, Mabwell (Shanghai) Bioscience Co Ltd |
君迈康 |
Mainland China |
Psoriasis; Arthritis, Rheumatoid; Spondylitis, Ankylosing |
Shanghai Junshi Biosciences Co Ltd |
2022-03-01 |
Arthritis, Rheumatoid; Spondylitis, Ankylosing; Pediatric Crohn's disease; Arthritis, Juvenile; Psoriasis; Uveitis; Crohn Disease |
Details
|
| Adalimumab biosimilar (Samsung Bioepis) |
SB-5 |
Approved |
Samsung Bioepis Co Ltd |
Imraldi, Hadlima |
EU |
Uveitis; Hidradenitis Suppurativa; Arthritis, Psoriatic; Spondylitis, Ankylosing; Arthritis, Rheumatoid; Colitis, Ulcerative; Crohn Disease; Plaque psoriasis; Pediatric Crohn's disease; Arthritis, Juvenile; Psoriasis; Pediatric ulcerative colitis |
Samsung Bioepis Nl Bv |
2017-08-24 |
Arthritis, Rheumatoid; Spondylitis, Ankylosing; Pediatric Crohn's disease; Arthritis, Juvenile; Psoriasis; Pediatric ulcerative colitis; Arthritis, Psoriatic; Colitis, Ulcerative; Uveitis; Hidradenitis Suppurativa; Crohn Disease; Plaque psoriasis |
Details
|
| Adalimumab biosimilar (Fujifilm Kyowa Kirin Biologics/Mylan) |
FKB-327 |
Approved |
Fujifilm Kyowa Kirin Biologics Co Ltd |
Hulio |
EU |
Colitis, Ulcerative; Crohn Disease; Uveitis; Arthritis, Psoriatic; Hidradenitis Suppurativa; Spondylitis, Ankylosing; Arthritis, Rheumatoid |
Biosimilar Collaborations Ireland Ltd |
2018-09-16 |
Arthritis, Rheumatoid; Spondylitis, Ankylosing; Colitis, Ulcerative; Arthritis, Psoriatic; Uveitis; Hidradenitis Suppurativa; Crohn Disease |
Details
|
| Etanercept biosimilar (Sunshine Guojian) |
301S |
Approved |
Sunshine GuoJian Pharmaceutical (Shanghai) Co Ltd, Cipla Gmbh |
Yisaipu, 益赛普, Etanar, Etacept |
Mainland China |
Arthritis, Rheumatoid; Psoriasis; Spondylitis, Ankylosing |
Sunshine GuoJian Pharmaceutical (Shanghai) Co Ltd |
2005-06-20 |
Non-radiographic axial spondyloarthritis; Arthritis, Rheumatoid; Spondylitis, Ankylosing; Psoriasis |
Details
|
| PG-201 |
PG-201 |
Approved |
Viromed |
Layla |
South Korea |
Osteoarthritis |
Pmg Pharm Co Ltd |
2012-01-01 |
Osteoarthritis, Knee; Osteoarthritis |
Details
|
| Adalimumab biosimilar (Sandoz) |
GP-2017 |
Approved |
Sandoz |
Hefiya, Hyrimoz, Halimatoz |
EU |
|
Sandoz Gmbh |
2018-07-26 |
Non-radiographic axial spondyloarthritis; Polyarticular Juvenile Idiopathic Arthritis; Arthritis, Rheumatoid; Spondylitis, Ankylosing; Arthritis, Juvenile; Psoriasis; Arthritis, Psoriatic; Colitis, Ulcerative; Uveitis; Hidradenitis Suppurativa; Crohn Disease; Plaque psoriasis |
Details
|
| Edaravone/(+)-2-Decanol |
SIM-071201; Y-2 |
Approved |
Jiangsu Simcere Pharmaceutical Co Ltd |
Sanbexin, 三贝心, 三贝欣 |
Mainland China |
Stroke |
Nanjing Simcere Dongyuan Pharmaceutical Co Ltd |
2020-07-29 |
Intracranial Hemorrhages; Brain Infarction; Cerebral Hemorrhage; Stroke; Alzheimer Disease; Cognitive Dysfunction; Amyotrophic Lateral Sclerosis; Cognition Disorders |
Details
|
| Adalimumab biosimilar (CTTQ Pharma) |
TQZ-2301; TQZ2301 |
Approved |
Jiangsu Chia Tai-Tianqing Pharmaceutical Co Ltd |
|
Mainland China |
Spondylitis, Ankylosing; Arthritis, Rheumatoid |
Jiangsu Chia Tai-Tianqing Pharmaceutical Co Ltd |
2022-01-18 |
Arthritis, Rheumatoid; Spondylitis, Ankylosing; Arthritis, Juvenile; Psoriasis; Colitis, Ulcerative; Arthritis, Psoriatic; Crohn Disease |
Details
|
| Infliximab biosimilar (AXXO) |
|
Approved |
Axxo |
|
Russian Federation |
Crohn Disease; Autoimmune Diseases |
Axxo |
2013-01-01 |
Autoimmune Diseases; Crohn Disease |
Details
|
| Infliximab biosimilar (Celltrion) |
CT-P13 |
Approved |
Celltrion Inc |
ZYMFENTRA, Remsima, Flammegis, Inflectra, Inflectra/Remsima |
EU |
Spondylitis, Ankylosing; Arthritis, Rheumatoid; Colitis, Ulcerative; Crohn Disease; Psoriasis; Arthritis, Psoriatic |
Celltrion Healthcare Hungary Kft |
2013-09-10 |
Non-radiographic axial spondyloarthritis; Inflammatory Bowel Diseases; Pediatric Crohn's disease; Spondylitis, Ankylosing; Arthritis, Rheumatoid; Psoriasis; Arthritis, Psoriatic; Colitis, Ulcerative; Hidradenitis Suppurativa; Crohn Disease; Plaque psoriasis |
Details
|
| Adalimumab biosimilar (Zydus Cadila) |
ZRC-3197 |
Approved |
Zydus Cadila |
Exemptia, Adaly |
India |
Arthritis, Juvenile; Arthritis, Rheumatoid; Arthritis, Psoriatic; Spondylitis, Ankylosing |
Zydus Cadila |
2014-12-09 |
Arthritis, Rheumatoid; Spondylitis, Ankylosing; Arthritis, Juvenile; Arthritis, Psoriatic |
Details
|
| Ozoralizumab |
PF-5230896; ATN-103; PF-05230896; TS-152 |
Approved |
Ablynx Nv |
ナノゾラ, Nanozora |
Japan |
Arthritis, Rheumatoid |
Taisho Pharmaceutical Co Ltd |
2022-09-26 |
Arthritis, Rheumatoid |
Details
|
| Opinercept |
E-11; ENIA-11 |
Approved |
Tsh Biopharm, Mycenax Biotech Inc |
TuNEX |
Taiwan |
Arthritis, Rheumatoid |
null |
2017-01-01 |
Non-radiographic axial spondyloarthritis; Arthritis, Rheumatoid |
Details
|
| Adalimumab biosimilar (Hisun Pharma) |
HS-016 |
Approved |
Zhejiang Hisun Pharmaceutical Co Ltd |
安健宁 |
Mainland China |
Arthritis, Rheumatoid; Spondylitis, Ankylosing |
Hisun Biopharmaceutical Co Ltd |
2019-12-06 |
Arthritis, Rheumatoid; Spondylitis, Ankylosing |
Details
|
| Infliximab biosimilar (Merck & Co/Samsung Bioepis) |
SB-2 |
Approved |
Samsung Bioepis Co Ltd, Merck & Co Inc |
Renflexis, Flixabi |
EU |
Crohn Disease; Psoriasis; Arthritis, Psoriatic; Colitis, Ulcerative; Spondylitis, Ankylosing; Arthritis, Rheumatoid |
Samsung Bioepis Nl Bv |
2016-05-26 |
Spondylitis, Ankylosing; Arthritis, Rheumatoid; Psoriasis; Arthritis, Psoriatic; Colitis, Ulcerative; Crohn Disease; Plaque psoriasis |
Details
|
| Andrographolide/Sodium Hydrogen Sulfite |
|
Approved |
|
|
|
|
|
|
Bacterial Infections |
Details
|
| Adalimumab biosimilar (Coherus BioSciences) |
CHS-1420 |
Approved |
Coherus Biosciences Inc |
Yusimry |
United States |
Colitis, Ulcerative; Spondylitis, Ankylosing; Crohn Disease; Plaque psoriasis; Arthritis, Juvenile; Arthritis, Rheumatoid; Arthritis, Psoriatic |
Coherus Biosciences Inc |
2021-12-17 |
Spondylitis, Ankylosing; Arthritis, Rheumatoid; Arthritis, Juvenile; Psoriasis; Colitis, Ulcerative; Arthritis, Psoriatic; Crohn Disease; Plaque psoriasis |
Details
|
| Infliximab biosimilar (Nichiiko/Aprogen) |
NI-071; GS-071; GS-07; AP-07 |
Approved |
Nichi-Iko Pharmaceutical Co Ltd, Aprogen |
|
Japan |
Arthritis, Psoriatic; Arthritis, Rheumatoid; Colitis, Ulcerative; Crohn Disease; Psoriasis |
Nichi-Iko Pharmaceutical Co Ltd |
2017-09-27 |
Arthritis, Rheumatoid; Psoriasis; Arthritis, Psoriatic; Colitis, Ulcerative; Crohn Disease |
Details
|
| Thalidomide |
NSC-66847; NSC-527179; K-17; VP-02; FPF-300; FPF300 |
Approved |
Celgene Corp |
Talizer, Thalidomide Celgene, Thalidomide Pharmion, Synovir, Thalomid, Thaled |
Mainland China |
Leprosy, Lepromatous; Multiple Myeloma |
Changzhou Pharmaceutical Factory |
1982-01-01 |
HIV Wasting Syndrome; Angiodysplasia; Primary Myelofibrosis; Neuroectodermal Tumors, Primitive; Prostatitis; Colorectal Neoplasms; Osteosarcoma; Lymphoma, Mantle-Cell; Sarcoma, Ewing; Retinoblastoma; Erythema Nodosum; Drug Resistant Epilepsy; Xerostomia; Sarcoma; Pancreatitis, Chronic; Adenocarcinoma, Clear Cell; Lymphoma, Follicular; Arachnoiditis; Carcinoma, Adenosquamous; Gastrointestinal Hemorrhage; Cholangitis, Sclerosing; Prostatic Neoplasms; Pelvic Pain; Neoplasm Metastasis; Stomatitis; Burning Mouth Syndrome; Mycobacterium avium-intracellulare Infection; Amyotrophic Lateral Sclerosis; Melanoma; Myelodysplastic-Myeloproliferative Diseases; Carcinoma, Hepatocellular; Leukemia, Lymphocytic, Chronic, B-Cell; Vascular Malformations; Tuberculosis; Appendiceal Neoplasms; Lymphoma, Non-Hodgkin; Uterine Neoplasms; Anemia, Sideroblastic; Glioma; Leprosy, Lepromatous; Endometrial Neoplasms; Lung Neoplasms; Waldenstrom Macroglobulinemia; Kidney Neoplasms; Thalassemia; Carcinoid Tumor; Lupus Erythematosus, Discoid |
Details
|
| Adalimumab biosimilar (Pfizer) |
PF-06410293 |
Approved |
Pfizer Inc |
Abrilada, Fyzoclad, Amsparity |
United States |
Arthritis, Psoriatic; Spondylitis, Ankylosing; Psoriasis; Arthritis, Rheumatoid; Colitis, Ulcerative; Arthritis, Juvenile; Crohn Disease |
Pfizer Inc |
2019-11-15 |
Spondylitis, Ankylosing; Arthritis, Rheumatoid; Arthritis, Juvenile; Psoriasis; Arthritis, Psoriatic; Colitis, Ulcerative; Hidradenitis Suppurativa; Uveitis; Crohn Disease |
Details
|
| Etanercept biosimilar (Hisun-Pfizer Pharmaceuticals) |
|
Approved |
Hisun-Pfizer |
AnBaiNuo |
Mainland China |
Spondylitis, Ankylosing; Arthritis, Rheumatoid; Psoriasis; Arthritis, Psoriatic |
Wyeth Europa Ltd |
2010-02-26 |
Spondylitis, Ankylosing; Arthritis, Rheumatoid; Psoriasis; Arthritis, Psoriatic |
Details
|
| Adalimumab |
ABT-D2E7; LU-200134; D2E7 |
Approved |
Abbvie Inc |
Humira, Trudexa, 修美乐 |
United States |
Arthritis, Rheumatoid |
Abbvie Inc |
2002-12-31 |
Uveitis, Posterior; Glomerulosclerosis, Focal Segmental; Colitis, Ulcerative; Osteoarthritis, Knee; Cystitis, Interstitial; Arthritis, Psoriatic; Asthma; Arthritis; Uveitis; Hidradenitis Suppurativa; Mucopolysaccharidosis VI; Mucopolysaccharidosis II; Nephrotic Syndrome; Osteoarthritis; Crohn Disease; Diabetic Retinopathy; Choroidal Neovascularization; Plaque psoriasis; Panuveitis; Spondylarthritis; Pain; Prostatic Hyperplasia; Pyoderma Gangrenosum; Uveitis, Intermediate; Pouchitis; Arthritis, Rheumatoid; Pediatric Crohn's disease; Sarcoidosis; Non-radiographic axial spondyloarthritis; Spondylitis, Ankylosing; Coronavirus Disease 2019 (COVID-19); Dupuytren Contracture; Arthritis, Juvenile; Mucopolysaccharidosis I; Nephrosis, Lipoid; Psoriasis; Periarthritis |
Details
|
| Etanercept biosimilar (Qilu Pharmaceutical) |
QL-0902 |
Approved |
Qilu Pharmaceutical Co Ltd |
|
Mainland China |
|
Qilu Pharmaceutical Co Ltd |
2023-12-19 |
Arthritis, Rheumatoid; Spondylitis, Ankylosing |
Details
|
| Etanercept biosimilar (Samsung Bioepis) |
SB-4 |
Approved |
Samsung Bioepis Co Ltd |
Eticovo, Benepali |
EU |
Arthritis, Psoriatic; Arthritis, Rheumatoid; Spondylitis, Ankylosing; Plaque psoriasis |
Samsung Bioepis Nl Bv |
2016-01-13 |
Arthritis, Rheumatoid; Spondylitis, Ankylosing; Arthritis, Juvenile; Psoriasis; Arthritis, Psoriatic; Plaque psoriasis |
Details
|
| Etanercept |
TNR-001 |
Approved |
Amgen Inc, Pfizer Inc |
Lifmior, 恩利, Enbrel |
EU |
Plaque psoriasis; Spondylitis, Ankylosing; Arthritis, Rheumatoid; Arthritis, Psoriatic; Arthritis, Juvenile |
Pfizer Europe Ma Eeig |
2000-02-02 |
Obesity; Pulmonary Fibrosis; Psoriasis; Myositis, Inclusion Body; Prostatic Neoplasms; Temporomandibular Joint Disorders; Arthritis, Psoriatic; Bone Neoplasms; Lichen Planus; Alzheimer Disease; Asthma; Hidradenitis Suppurativa; Respiratory Distress Syndrome, Adult; Inflammation; Cachexia; Arthritis; Lung Neoplasms; Uveitis; Plaque psoriasis; Vitiligo; Neoplasm Metastasis; Acute Lung Injury; Kidney Failure, Chronic; Stomatitis; Spondylitis, Ankylosing; Granulomatosis with Polyangiitis; Leukemia; Non-radiographic axial spondyloarthritis; Bronchiolitis Obliterans; Solid tumours; Tinnitus; HIV Infections; Lupus Erythematosus, Discoid; Rejection of lung transplantation; Arthritis, Rheumatoid; Fanconi Anemia; Diabetes Mellitus, Type 1; Vasculitis; Graft vs Host Disease; Lupus Erythematosus, Cutaneous; Synovitis; Sjogren's Syndrome; Anorexia; Metabolic Syndrome; Spondylarthritis; Chronic Urticaria; Small Cell Lung Carcinoma; Arthritis, Juvenile |
Details
|
 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free! Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!  Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!