 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!
 Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!  Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!
> GMP 등급 사이토카인
세포치료제를 제조할 때, 핵심 원자재로서 일관된 GMP 제품을 사용하는 것은 매우 중요합니다. ACROBiosystems는 규제 지침을 준수하면서 안전하고 신뢰할 수 있으며 생물활성이 검증된 고품질의 GMP 제품을 제공하기 위해 최선을 다하고 있습니다.

ACROBiosystems의 GMP 사이토카인과 성장 인자를 사용하여, 걱정없이 세포치료제 제조 공정을 개발하십시오. 당사는 단백질 개발 및 제조에 있어서 전문적이고 풍부한 경험이 있으며, 엄격한 품질 관리 및 규제 서류 지원을 통해 업계 최고의 GMP 단백질을 제공합니다. 또한, 당사의 일부 GMP 단백질은 비GMP 단백질과 동일한 클론, 서열, 발현 시스템을 이용하였기 때문에 추후 비GMP 등급 원료를 GMP 등급 원료로 빠르고 쉽게 전환할 수 있습니다.

고품질의 GMP 제품을 합리적인 비용으로 제공한다면, 세포치료제를 포함한 혁신적인 의약품 개발에 대한 접근 장벽을 낮출 수 있습니다. 당사의 새로운 GMP 제조 시설은 GMP 등급의 단백질, 효소, 활성화 비드 및 기타 원료 생산을 위해 특별히 설계되었습니다.
Want to learn more about
your GMP Protein options?
당사의 GMP 제품은 귀하의 요구사항을 충족하고 규정을 준수하기 위하여, 대용량 중간 생성물부터 최종 동결건조 제품에 이르기까지 전체 생산 과정에서 품질 관리 테스트를 진행합니다. 당사는 포괄적인 품질 관리 시스템을 통해 모든 GMP 제품에서 생물활성, 일관성 및 안정성을 보장합니다.
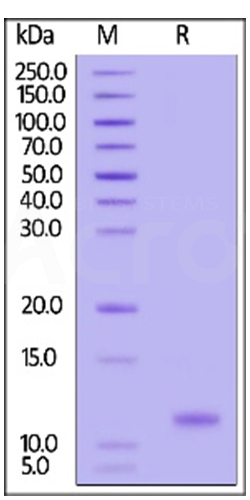
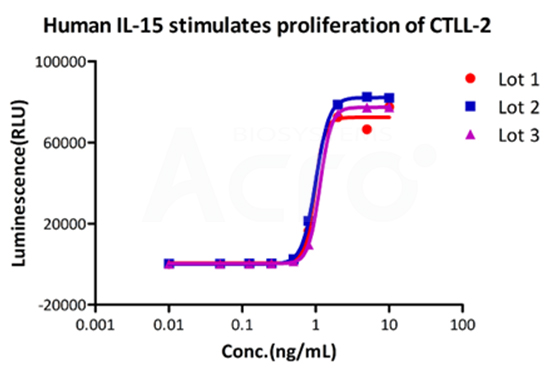
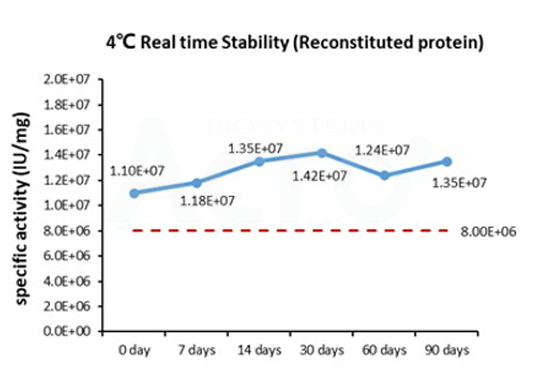
Three independent lots of GMP Human IL-15 (Cat. No. GMP-L15H13) were tested for the ability to simulate the proliferation of CTLL-2 cells. Average specific activity of GMP Human IL-15 was defined to be more than 0.8 x 107 IU/mg after calibration against human IL-15 WHO International Standard (NIBSC code: 95/554).
Human IL-2, GMP-grade
Human IL-7, GMP-grade
Human IL-15, GMP-grade
Explore >>
Request a protocol for GMP-grade IL-15 Bioactivity Verification
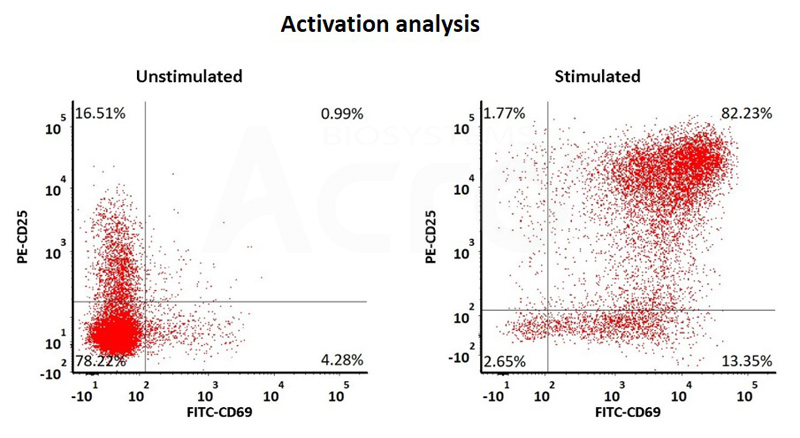
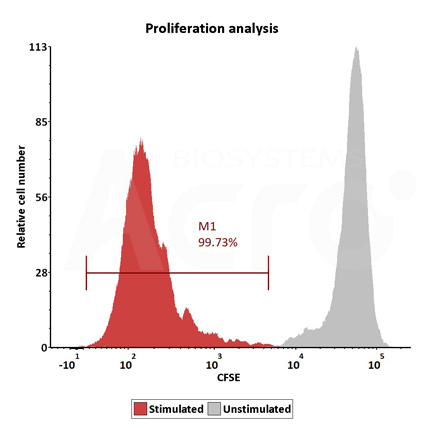
Human T cells were stimulated using GMP-grade ActiveMax Human T Cell Activation / Expansion CD3/CD28 beads (Cat. No. GMP-MBS001 ) for 24 hours. Activation was assessed by measuring expression of both activation markers CD25 and CD69 expression on T cell surface by staining with PE labeled anti-human CD25 antibody and FITC-labeled anti-human CD69 antibody respectively (QC tested).
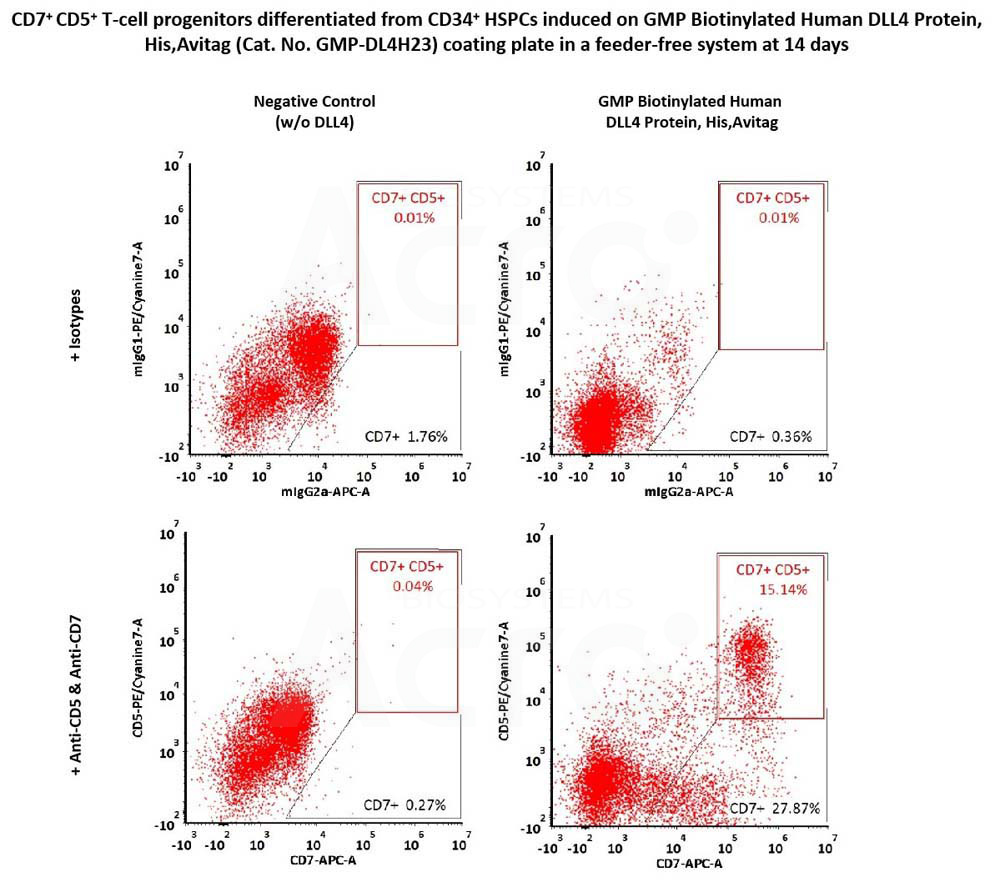
CD34+ CD45+ hematopoietic cells were seeded on GMP Biotinylated Human DLL4 Protein, His,Avitag (Cat. No. GMP-DL4H23) coated plates and differentiated for 14 days, then flow cytometry was used to detect the expression of T-cell progenitor markers, CD7 and CD5. The GMP Biotinylated Human DLL4 Protein, His,Avitag together with SCF, TPO, Flt3L and IL7, could induce the formation of CD7+ and CD7+ CD5+ T-cell progenitors (Routinely tested).
DLL4, Fc tag, GMP-grade
Biotinylated DLL4, His, Avitag, GMP-grade
VCAM-1, GMP-grade
Explore >>

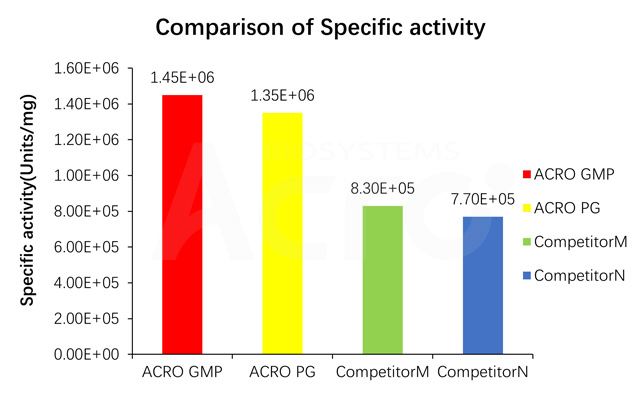
Specific activity for GMP GENIUS Nuclease is measured under standard assay conditions. The specific activity of GMP GENIUS Nuclease, is >1.2E+06 unit/mg protein. One unit will digest sonicated salmon sperm DNA to acid-soluble oligonucleotides equivalent to a ΔA260 of 1.0 in 30 min at pH 8.0 at 37 °C, which corresponds approximately to complete digestion of 37 μg DNA.
Salt-Active GENIUS Nuclease, GMP-grade
NLS-Cas9 Nuclease, GMP-grade
Explore >>
Interested in testing
a GMP grade product ?
적용 분야별로 귀하에게 필요한 GMP 제품을 찾을 수 있습니다! T 세포, NK 세포 및 수지상 세포(DC)의 세포 배양에 필요한 다양한 제품들이 준비되어 있습니다. 또한 DLL4, VCAM-1 등과 같은 iPSC 성장 인자 및 분화 인자 신제품이 계속 출시되고 있습니다. 다양한 종류의 기저막 추출물(제품명: Mogengel Matrix) 및 세포외 기질 단백질도 이용할 수 있습니다.
필요한 GMP 제품이 있습니까? 단백질 이름을 검색하면, 원하는 GMP 제품을 찾을 수 있습니다. 당사는 세포치료제 제조 공정에 필요한 원료에 대한 규제 지침을 준수하여 GMP 제품을 제조합니다. 한편, 제품명에 Premium Grade가 명시된 제품은 추후 GMP로의 효율적인 전환을 위해 당사에서 특별히 제작한 Pre-GMP(준GMP등급) 제품입니다.
당사의 GMP 평가항목, 품질 및 규제 서류 지원 알아보기 
| Molecule | Source | GMP Grade Catalog No. | DMF Filed for GMP Grade | Premium (Pre-GMP) Grade Catalog No. |
|---|
*Already purchased a DMF-filed protein? Click here to apply for your DMF authorization.
Can’t find the
GMP Product you need?

이 15분의 웨비나를 통해, 귀하가 가장 중시하는 환자 안전, 배치 간 일관성 및 공급 신뢰성을 보장하기 위하여, 올바른 원료 공급업체를 선택하는 것이 왜 중요한지 확인해 보세요.
ACROBiosystems는 세포치료제 및 유전자치료제의 임상 연구 및 상업화에 사용할 고품질의 핵심 원료를 개발하기 위해 전념하고 있습니다. 현재의 의약품 제조 규칙과 더 엄격한 품질 관리 및 제품 출고 시험 요건을 준수함으로써, 당사는 고객에게 최고의 GMP 제품을 제공하기 위해 노력하고 있습니다. 당사는 GMP 품질 관리 시스템을 지속적으로 개선하여, 세포치료제 및 유전자치료제 제조에 대한 국제 규제 요구 사항을 충족하는 더 우수한 GMP 제품을 생산합니다. 당사는 모든 고객의 실사(Audit) 요청을 환영하며, 정기적으로 자체 시설을 감사하여 준수 여부를 확인하고 있습니다.
각 GMP 단백질은 GMP 준수 시설에서 제조 및 검증 됩니다. 당사의 GMP 품질 관리 시스템은 관련 cGMP 및 GMP 지침에 따라 개발 및 유지 관리 되고 있습니다.
주요 내용은 다음과 같습니다:
각 GMP 단백질은 GMP 준수 시설에서 제조 및 검증 됩니다. 당사의 GMP 품질 관리 시스템은 관련 cGMP 및 GMP 지침에 따라 개발 및 유지 관리 되고 있습니다.
Want to learn more about
your GMP Protein options?
세포치료제 및 유전자치료제의 완제약약품 내에 존재하는 위험 물질 오염은 중대한 문제입니다. 따라서 외부 오염을 최소화하기 위해서 엄격한 품질 관리 시스템이 필요합니다. 당사의 GMP 제품은 규제 기관에서 발표한 여러 GMP 가이드라인을 준수하며, 생산 및 품질 관리 과정에서 주기적인 감사를 진행하고 있습니다.
GMP 가이드라인 세부내용:
당사의 숙련된 규제 지원(Regulatory Support)팀은 중국, 미국, 한국 등 글로벌 치료제 제조사와 협력한 경험이 있습니다. 당사의 도움으로 다수의 규제 서류가 CDE, FDA 및 KFDA에 제출되었고 승인된 바 있습니다. 고객이 IND 신청을 위해 규제 서류를 요청할 때, 당사는 광범위한 규제 지원 파일(RSF) 문서를 제공해드립니다.
Learn More About our GMP Facilities & Capabilities 
Want to learn more about
your GMP Protein options?
RUO 단백질에서 GMP 단백질로의 전환을 준비하는 데 있어 언제 전환을 시작해야 할지 정확히 알기 어려울 수 있습니다. GMP 원료를 활용하는 규제 환경에 대한 통찰력을 얻고, 임상 단계에 수월하게 진입하는 데 도움이 되는 몇 가지 질문들을 소개 드립니다.
Learn more about making the transition to GMP here!
당사는 사전에 수립된 공급업체 자격 조건 및 모니터링 절차에 따라 적격 승인된 공급업체로부터만 원료를 구매합니다. ACROBiosystems는 당사의 원료 공급업체와 함께 당사의 GMP 시설에서 사용되는 원료에 대해 매년 위험성 평가를 수행합니다. 즉, 분석 인증서(CoA), 원산지 증명서(CoO), TSE/BSE 미감염 증명서 및 동물유래물질 불포함 증명서 등 관련 문서를 검토하고 보관합니다. 핵심 원료는 검증 및 인증이 된 후에야 비로소 GMP 시설에서 사용될 수 있습니다.
세포치료제 제조기업은 가능한 한 의약품 등급 또는 GMP 등급 제품을 구매하는 것이 좋습니다. GMP 제품은 관련 국제 표준에 따라 생산되고 테스트 됩니다. 또한, 원료 공급업체는 ISO 9001 / 13485 인증과 함께 인증된 품질 관리 시스템(QMS)을 갖추고 있어야 합니다. 즉, 고객의 기대에 부합하는 품질을 보장하기 위해 독립적인 감사, 품질 정책 및 품질 프로세스가 수립되어야 합니다. 아울러, 세포치료제 제조기업은 설문지, 정시 배송, SCAR 및 기타 감사 활동을 통해 공급업체를 검증하는 등의 실사를 수행해야 합니다. 각 원료 공급업체에게 분석 인증서, 원산지 증명서 및 기타 감사 결과 문서 요청한 후, 서류를 검토하고, 모든 문서들을 보관해야 합니다.
세포치료제 제조기업은 생산에 들어갈 때 문제가 발생하지 않도록 가능한 한 빨리 잠재적 원료 공급업체와 논의를 시작해야 합니다. 예를 들어, 원료 공급업체의 생산 규모 확대 능력을 확실히 한다면, 막판 변수들을 사전 방지함으로써 비용이 많이 드는 2차 검증 연구를 피할 수 있습니다.
또한, 플랜B 공급업체를 확보해 두는 것도 매우 중요합니다. 원료들이 동일해 보일 수 있지만 인체 안에서 어떻게 작동할 지를 보장할 수는 없습니다. 게다가 원료 공급업체를 변경한다는 것은 쉬운 일이 아닙니다. 서로 다른 공급업체의 원료들 간에 동등성을 보여주는 후속 검증 연구가 필요하기 때문입니다. 따라서, 1차 공급업체와 문제가 발생했을 때에 대비하여, 플랜B 공급업체를 마련해 두는 보험이 필요합니다.
*International standards include:
제조된 세포치료제 및 유전자치료제의 품질은 생산에 사용된 원료에 의해 직접적인 영향을 받으며, 이는 결국 치료제의 영향과 효능에 영향을 미칩니다. 관련 규정에 따르면, 원료 선택 시 그 필요성, 합리성, 안전성을 고려해야 하며, 가급적 인체용으로 승인된 물질을 선택하거나 약전 표준을 충족하는 물질을 선택하는 것이 좋습니다. 초기 전임상 단계에서는 원료에 대한 안전 및 품질 요구사항의 우선순위가 낮지만, CMC 또는 임상 전환 중에는 규제를 준수하는 GMP 등급 원료로의 전환이 필수적입니다. 이러한 전환에는 철저한 테스트, 안전성 평가 및 프로세스 검증이 포함되며 상당한 시간과 노력이 필요합니다.
동일한 원료 공급업체를 활용하는 경우, 프리미엄 등급(PG) 원료와 GMP 등급 원료간 차이는 주로 규제 지원 문서화에 있을 뿐, 이상적으로는 프리미엄 등급(PG) 원료의 물리적 특성 및 생물학적 활성은 GMP 등급의 원료와 유사합니다. 환자와 상호 작용하는 원료에 대한 엄격한 안전성 테스트가 중요하기 때문에, PG와 GMP 원료 간의 제조 공정의 일관성을 확인하는 것이 좋습니다. ACROBiosystems는 'PG와 GMP 제품을 생산할 때, 둘 다 동일한 세포 은행에서 세포주를 공급받고, 엄격한 품질 관리를 거치며, 광범위한 테스트들을 통해 생물활성을 검증했다'는 것을 보장합니다.
한편, 임상 적용의 경우, 전임상 단계부터 GMP로 사전 전환하는 것을 추천합니다. 보다 관리 가능한 단계에서 동등성 테스트를 용이하게 할 수 있기 때문입니다. 또한, 품질 관리, 단백질 생화학, 분석 테스트 및 규제 문제에 정통한 숙련된 공급업체를 선택하는 것이 유리합니다. 경험이 풍부한 공급업체는 변화하는 규제 환경을 탐색하는 데 도움을 주며, 규제 프로세스를 간소화하기 위해 의약품 마스터 파일(DMF) 같은 필수 문서를 제공합니다. 가급적이면 직접 또는 온라인으로 공급업체 시설에 대한 감사를 실시하여 공급업체와 고객 간의 전략적 파트너십을 강화하는 것이 좋습니다. ACROBiosystems는 최소한 3개의 연속 배치에 대해 생산 절차 규정을 엄격하게 준수함으로써, GMP 제품 라인을 통해 개발된 PG 등급 제품의 일관성과 QC 결과를 보장합니다
생물학적 시약은 가변성, 특히 사이토카인과 성장 인자에 대한 민감성 측면에서 악명 높습니다. 따라서, 이러한 시약은 안정적인 공정 구축 및 엄격한 적합성 평가를 거치고 나서, 엄격한 사양을 충족하는 여러 번의 연속 배치 테스트까지 통과한 후, 성공적인 개발 및 검증이 완료된 후에야 비로소 제품이 대규모 생산 및 상업화될 수 있습니다. 이러한 복잡하고 까다로운 과정 덕분에 GMP 원료의 일관성과 신뢰성이 보장될 수 있는 것입니다.
한편, GMP 제품 생산은 GMP 시설 및 GMP 품질 시스템 내에서 이루어지며, 문서화된 표준 운영 절차(SOP) 및 훈련된 전문 직원을 통해 구현됩니다. 물론 이러한 중요한 정보는 감사 중에 공개되어야 합니다. 재현 가능한 단백질을 일관되게 생산하는 공급업체의 능력을 평가하기 위해 여러 배치에서 데이터를 요청하는 것이 합리적입니다. 이상적으로는 전체 시스템에서 일관성을 평가하기 위해 다양한 배치에서 재료를 샘플링해야 합니다. ACROBiosystems는 표준 배치를 설정하고, 각 새로운 생산 배치를 시장에 출시하기 전에 사전에 설정된 표준 배치와 비교합니다. 이러한 접근 방식 덕분에, 배치간 변동성을 완화시키고 일관된 제품 성능을 보장할 수 있습니다. 현재 당사는 특히 생물활성 면에서 배치 간 차이 10-15% 범위를 유지함으로써, GMP 제품의 배치 간 높은 동일성을 보장하고 있습니다.
완제의약품의 품질을 확보하기 위해서는 세포치료제 및 유전자치료제 제조에 사용되는 모든 원료에 대한 안전성이 매우 중요합니다. 특히, 외인성 요인에 의한 오염을 방지하기 위한 안전성 관리가 필요합니다. 이러한 '외인성' 요인은 접종원, 세포 기질, 공정 잔류물, 박테리아, 곰팡이, 마이코플라스마, 바이러스 등이 있습니다.
FDA는 최고 품질의 재료를 사용할 것을 권고하고 있으며, 이는 모든 원료 공급업체가 환자에 대한 불합리한 위험을 줄이기 위해서 원료 또는 시약이 적절한 품질 표준에 따라 생산되도록 보장해야 한다는 것을 의미합니다. 즉, CGT 생산에 사용되는 원료의 엄격한 품질 관리는 외부 오염 위험을 줄이고 세포치료제 및 유전자치료제의 안전성과 효능을 보장하기 위해 필요한 조치라는 뜻입니다. 이 요구 사항을 확장하여 다음과 같은 요구 사항이 다양한 국제 약전에 요약되어 있습니다:
Making the Transition to GMP Brochure 
The Balancing Act: Why Finding the Right Time to Introduce GMP Is Your Secret to Success 
Quality Management and Safety Evaluations for GMP 
Understanding Global GMP Regulatory Guidelines 
GMP Regulations Frequently Asked Questions 
Infographic - Production Process & Quality Control Workflow 
Qualifying Raw Materials for Cell Therapy Manufacturing 
Special Topic on Deep Interpretation of GMP Product Quality 1 
Looking for your next
GMP Supplier?
This web search service is supported by Google Inc.
