 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!
 Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!  Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!
| Cat. No. | Components | Size |
| GMP-CM3101 | CelThera™ GMP T Cell Expansion Medium | 1000mL |
| GMP-CM3101-1 | CelThera™ GMP T Cell Expansion Supplement | 7.25mL |

Compared to traditional culture media or xeno-free culture media, animal origin-free culture media can better reduce the risk of introducing potential pathogenic microorganisms during culture process, improve batch-to-batch consistency, and prevent T cell overactivation by undefined components in the serum.
CelThera™ GMP T Cell Expansion Medium does not require the addition of any serum or serum replacements and also maintains the high fold expansion of T cells. If users choose to add serum or serum replacement, the dosage should be determined by specific T cell applications.
The CelThera™ GMP T Cell Expansion Medium is stable for 18 months when stored under 2-8°C, protect from light.
The CelThera™ GMP T Cell Expansion Supplement is stable for 12 months when stored under -20°C or below, protect from light.
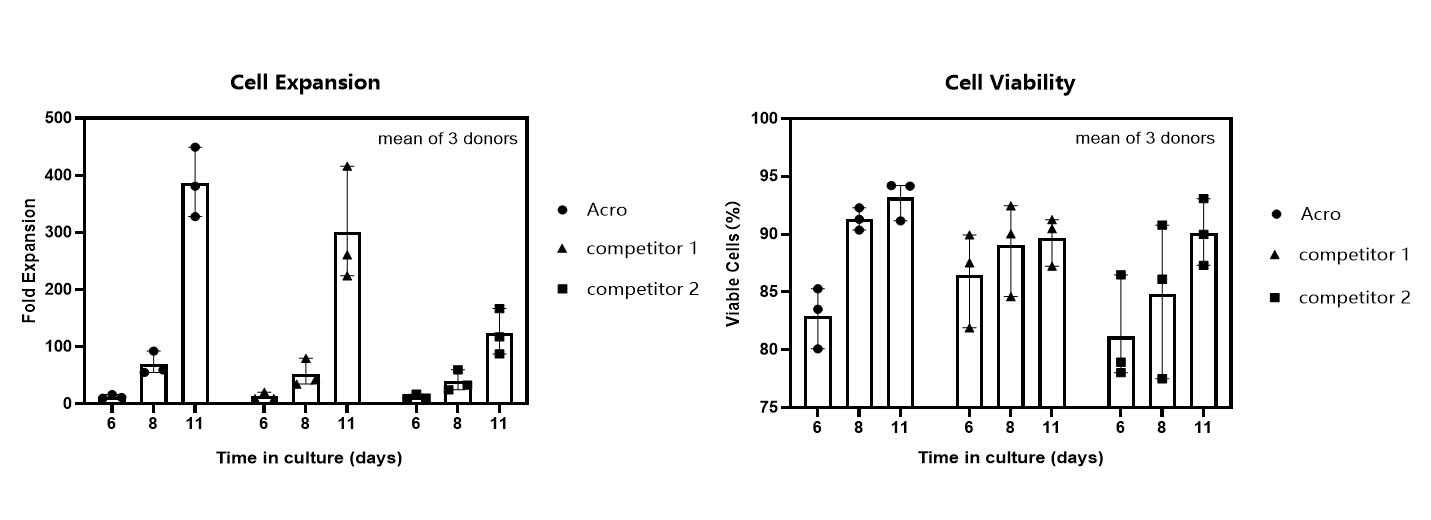
T cell expansion rate and cell viability in various media.
T cells from PBMCs of 3 different donors were activated and cultured for 11 days in various media supplemented with 300 IU/ml Acro GMP IL-2 (Cat. No. GMP-L02H14). Cell count and viability were performed on day 6, day 8 and day 11 by trypan blue staining. It indicated that cells in Acro T cell medium (Cat. No. GMP-CM3101) had a faster proliferation rate and higher viability than that of the other two media.
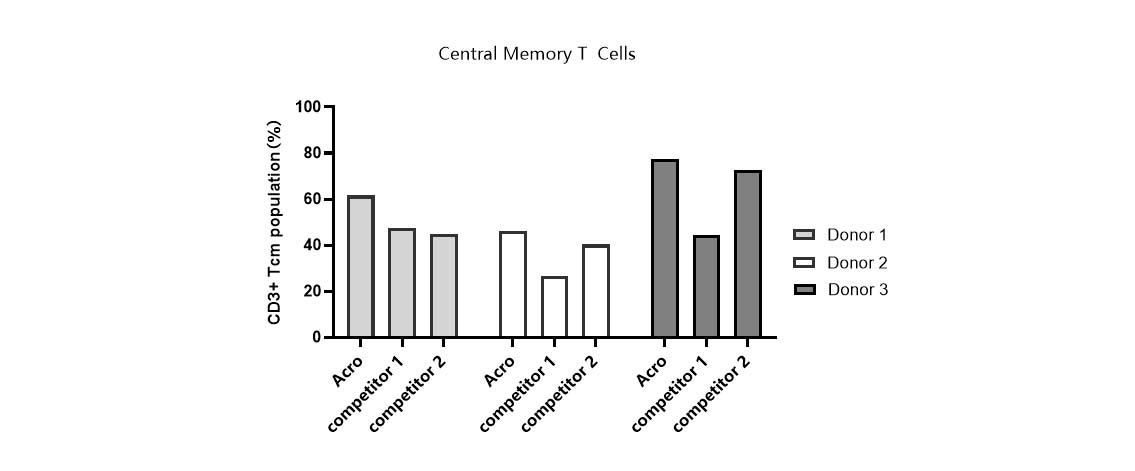
Tcm ratio in various media.
T cells from PBMCs of 3 different donors were activated and cultured in various media supplemented with 300 IU/ml Acro GMP IL-2 (Cat. No. GMP-L02H14). Tcm percentage (CD45RO+/CCR7+) was determined by flow cytometry when cells reached about 50-fold expansion. It indicated that cells in Acro T cell medium (Cat. No. GMP-CM3101) had a higher percentage of central memory T cells than that of the other two media.
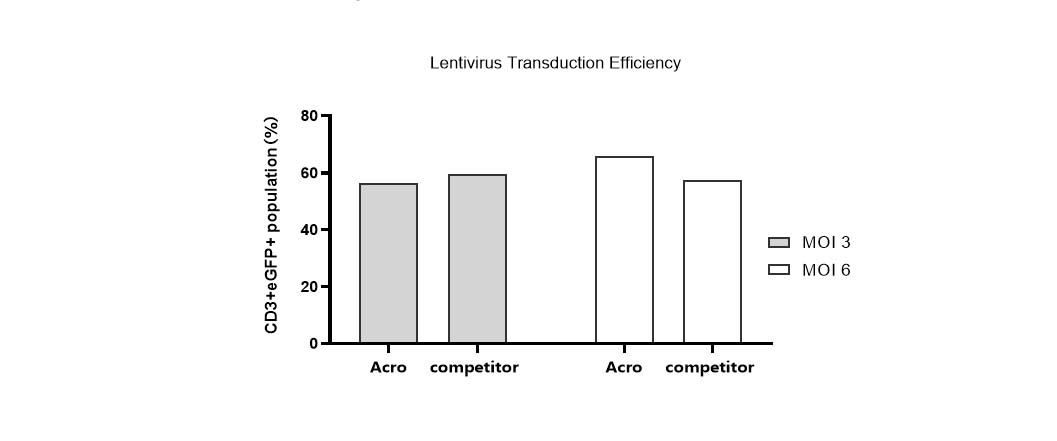
Lentivirus transduction efficiency in various media.
T cells from PBMCs were activated and cultured in various media supplemented with 300 IU/ml Acro GMP IL-2 (Cat. No. GMP-L02H14). 24 hours after activation, the cells were transduced with pLenti-CMV-EGFP-puro lentivirus (MOI=3 or 6). 24hrs after transduction, the lentivirus was removed by centrifugation. Then, the cells were cultured for 48hrs and the CD3+eGFP+ population was detected by flow cytometry. It indicated that cells in Acro T cell medium (Cat. No. GMP-CM3101) had a similar lentiviral transduction efficiency to that of the other medium.
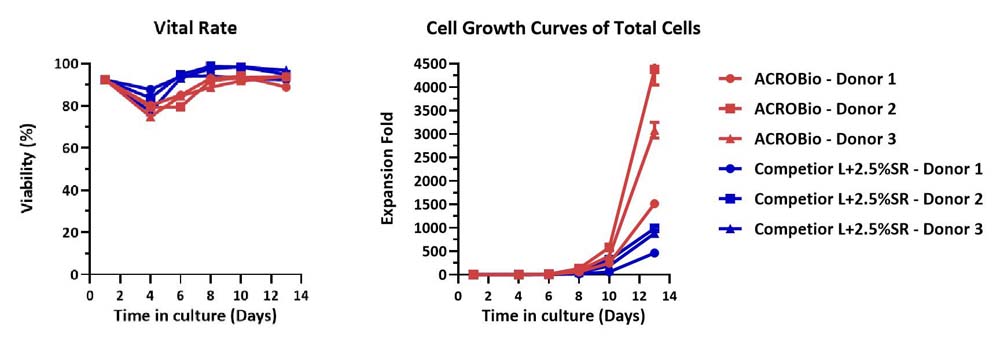
Human PBMCs were cultured with GMP Human IL-2 Protein (ACROBiosystems, Cat. No. GMP-L02H14) with CelThera™ GMP T Cell Expansion Medium (ACROBiosystems, Cat. No. GMP-CM3101) or T cell culture medium (Competitor L +2.5% SR) for two weeks. The result shows that CelThera™ GMP T Cell Expansion Medium (ACROBiosystems) can be comparable to Competitor L +2.5% SR. Notably, the cells exhibit better expansion in CelThera™ GMP T Cell Expansion Medium (ACROBiosystems, Cat. No. GMP-CM3101).
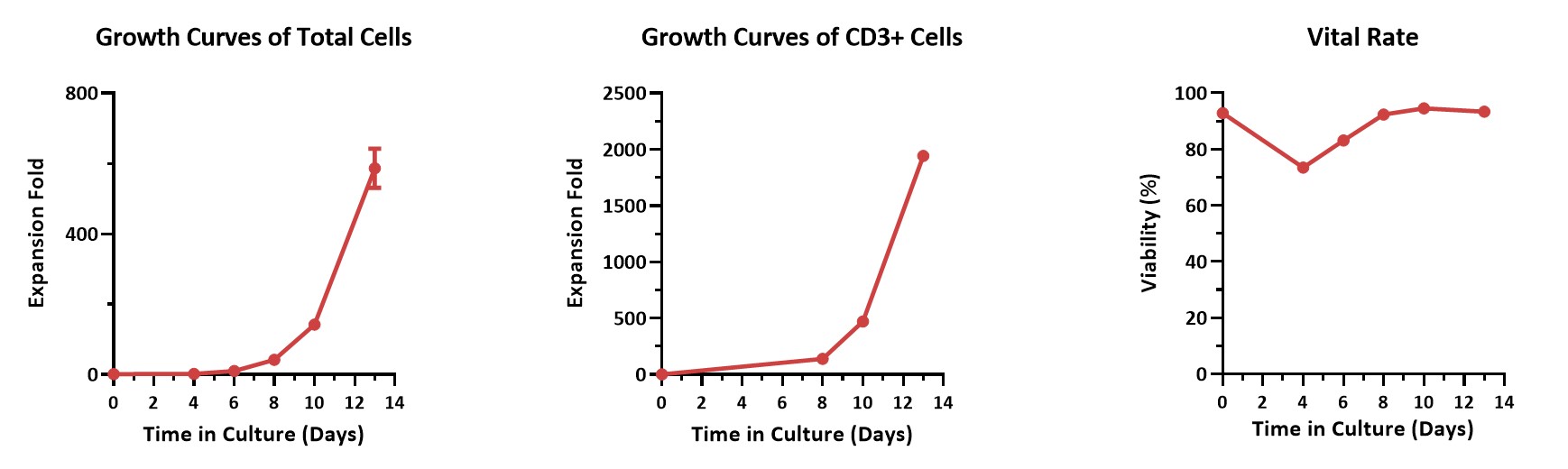
Human PBMCs were activated using 0.2 µg/mL GMP Monoclonal Anti-Human CD3 Antibody (OKT3) (ACROBiosystems, Cat. No. GMP-MC0323) and 1 µg/mL GMP Monoclonal Anti-Human CD28 Antibody (ACROBiosystems, Cat. No. GMP-MC2824), cultured with CelThera™ GMP T Cell Expansion culture medium (ACROBiosystems, Cat. No. GMP-CM3101) supplemented with 500 IU/mL GMP Human IL-2 Protein (ACROBiosystems, Cat. No. GMP-L02H14) for two weeks. The results showed that GMP human IL-2 protein, GMP monoclonal anti-human CD3 antibody (OKT3), GMP monoclonal anti-human CD28 antibody, and CelThrea™ GMP T cell expansion medium could be used to culture T cells in a 3L large system. It can efficiently expand cells with high viability.
ACROBiosystems GMP grade mediums are produced under a quality management system and in compliance with relevant guidelines: Ph. Eur General Chapter 5.2.12 Raw materials of biological origin for the production of cell-based and gene therapy medicinal products; USP <1043> Ancillary Materials for Cell, Gene, and Tissue-Engineered Products; ISO 20399: 2022(E), Biotechnology — Ancillary materials present during the production of cellular therapeutic products and gene therapy products
ACROBiosystems Quality Management System Contents:
Designed and Manufactured under ISO 9001:2015 and ISO 13485:2016.
Animal-Free materials
Materials purchased from the approved suppliers by QA
ISO 5 clean room for filling
Qualified personnel
Quality-related documents review and approve by QA
Fully batch production and control records
Equipment maintenance and calibration
Validation of analytical procedures
Stability studies conducted
Comprehensive regulatory support files
Request For Regulatory Support Files(RSF)
ACROBiosystems provide rigorous quality control tests (fully validated equipment, processes and test methods) on our GMP grade products to ensure that they meet stringent standards in terms of purity, safety, activity and inter-batch stability, and each bulk QC lot mainly contains the following specific information:
pH
Sterility
Osmolality
Endotoxin Level
Functionality
Mycoplasma testing
Batch-to-batch consistency
ACROBiosystems GMP grade products are designed for research, manufacturing use or ex vivo use. CAUTION: Not intended for direct human use.
All products are warranted to meet ACROBiosystems Inc.’s (“ACRO”) published specifications when used under normal laboratory conditions.
ACRO DOES NOT MAKE ANY OTHER WARRANTY OR REPRESENTATION WHATSOEVER, WHETHER EXPRESS OR IMPLIED, WITH RESPECT TO ITS PRODUCTS. IN PARTICULAR, ACRO DOES NOT MAKE ANY WARRANTY OF SUITABILITY, NONINFRINGEMENT, MERCHANTABILITY OR FITNESS FOR ANY PARTICULAR PURPOSE.
NOT WITH STANDING ANY OTHER PROVISIONS OF THESE TERMS AND/OR ANY OTHER AGREEMENT BETWEEN ACRO AND PURCHASER FOR THE PURCAHSE OF THE PRODUCTS, ACRO’S TOTAL LIABILITY TO PURCHASER ARISING FROM OR IN RELATION TO THESE TERMS, AN AGREEMENT BETWEEN THE PARTIES OR THE PRODUCTS, WHETHER ARISING IN CONTRACT, TORT OR OTHERWISE SHALL BE LIMITED TO THE TOTAL AMOUNT PAID BY PURCHASER TO ACRO FOR THE RELEVANT PRODUCTS. IN NO EVENT WILL ACRO BE LIABLE FOR THE COST OF PROCUREMENT OF SUBSTITUTE GOODS.
The following terms are offered to you upon your acceptance of these End User Terms of Use of Product. By using this product, you indicate your acknowledgment and agreement to these End User Terms of Use of Product. If you do not agree to be bound by and comply with all of the provisions of these End User Terms of Use of Product, you should contact your supplier of the product and make arrangements to return the product.
The End User is aware that ACROBiosystems Inc. and its affiliate (“ACRO”) sell GMP grade products designed for research, manufacturing use or ex vivo use and not intended for human in vivo applications. The End User further agrees, as a condition of the sales of ACRO’s GMP grade products that: a) the End User will not use this GMP grade product in any procedure wherein the product may be directly or indirectly administered to humans, unless the End User has obtained, or prior to their use will have obtained, an Investigational New Drug (IND) exemption from the FDA and will use the product only in accordance with the protocols of such IND and of the Institutional Review Board overseeing the proposed research, or b) the End User will use the products outside of the United States in accordance with the protocols of research approved by the applicable review board or authorized ethics committee and regulatory agencies to which the End User is subject to in their territory.

ACROBiosystems는 생체 활성이 높은 균질한 CD3 δ/CD3 ε 및 CD3 γ/CD3 ε 단백질을 개발하여 이중특이 항체를 치료제로의 임상 개발을 가속시킵니다.

We offer a wide range of cell and gene therapy solutions starting from discovery to the clinic. Explore our wide range of proteins, antibodies, kits, and other assays to accelerate the development of your cell and gene therapy.

오가노이드 툴 박스는 약물 개발 프로젝트의 진행을 가속할 수 있는 레디메이드 오가노이드와 오가노이드 분화 툴 키트 및 다양한 서비스를 포함하는 오가노이드 솔루션 모음입니다.

고품질 GMP 등급 사이토카인 IL-15,IL-7, IL-21 등 제품은 신속한 임상/신약승인신청을 위해 개발된 것입니다.

50+ car-t의 인기 타겟을 커버하고, car 발현 검사를 위해 특별히 설계된 항원 단백질은 스트림 검증을 통해 고특이성 car 발현을 검출할 수 있으며, 고 배치간 일관성을 가지고 있다.pe/fi표기 단백질로 한 단계 염색하면 고특이성, 무 백그라운드로 car 발현 검사를 검사할 수 있습니다.

cd20, claudin18.2, cd133, gprc5d, ccr5, ccr8을 포함한 안정적이고 활성이 높은 전장 다중 막관통 단백질은 면역, elisa, spr, bli, 세포 실험, car 양성률 검사 등에 광범위하게 사용된다. vlp, 스케일제거제, nanodisc의 다양한 기술 플랫폼은 다중 막관통 단백질을 목표로 하는 약물 연구에 도움을 준다.

GMP 등급 사이토카인. 고품질 세포 활성화 및 확장 시약. 유전자 편집 시약, 효소 CAR 검사 시약 등 제품. 세포 및 유전자 요법 고객님에게 포괄적인 솔루션을 제공하고 초기 약물 발견에서 임상 연구에 이르기까지 모든 단계에서 귀하와 동반하겠습니다.

현재 이미 알려진 거의 대부분의 면역 체크 포인트 분자를 포함하여 다양한 레벨과 물종을 제공하여 고객이 선택할수 있도록 하고있다. 천연중합체 형식은 mals를 통해 검증되었고 생물활성은 elisa/spr/bli/facs 등을 통해 검증되었으며 또한 biotin/fitc 표지는 높은 처리량을 가진 항체를 선별하는데 편리하다.

ACROBiosystems는 ADC약물의 연구개발을 돕기 위해 노력하고 있으며, 다양한 인기 타겟에 서로 다른 종, 다른 라벨의 제품을 개발했으며, 높은 순도, 높은 친화력 등 특징을 가지고 있으며, 면역, 항체 선택, spr, 세포 활성 검사 및 기타 실험에 적합하고, 해당 protocol을 무료로 제공합니다.

Fc 수용체 단백질은 모든 분자를 포함하고 있을 뿐만 아니라, 일반적인 돌연변이와 바이오틴 표기 종류을 포함하고 있어 고객들이 단일 클론 항체 개발을 가속화할 수 있다.

포괄적으로 풍부한 사이토카인 표적 단백질은 HEK293 진핵생물 시스템에 의해 발현되며 천연 구조에 더 가깝다. 고순도는 SDS-PAGE/HPLC/MALS에 의해 검증된다. 높은 생물학적 활성이 ELISA/SPR/BLI에 의해 검증된다. 배치간 일관성이 우수하여 항체 면역, 스크리닝 및 품질 관리 과정에 적합하다.

Aneuro는 ACROBiosystems가 뇌과학 연구에 초점을 맞춘 제품 라인으로서 치료 및 진단 연구 단백질, PFFs와 재조합 신경 인자 등 뇌과학 연구 분야에 중요한 단백질 제품을 제공하여 뇌과학 연구에 도움이 된다.
This web search service is supported by Google Inc.
