 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!
 Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!  Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!
The kit is used for quantitative detection of residual E. coli DNA in biopharmaceutical production.
It is for research use only.
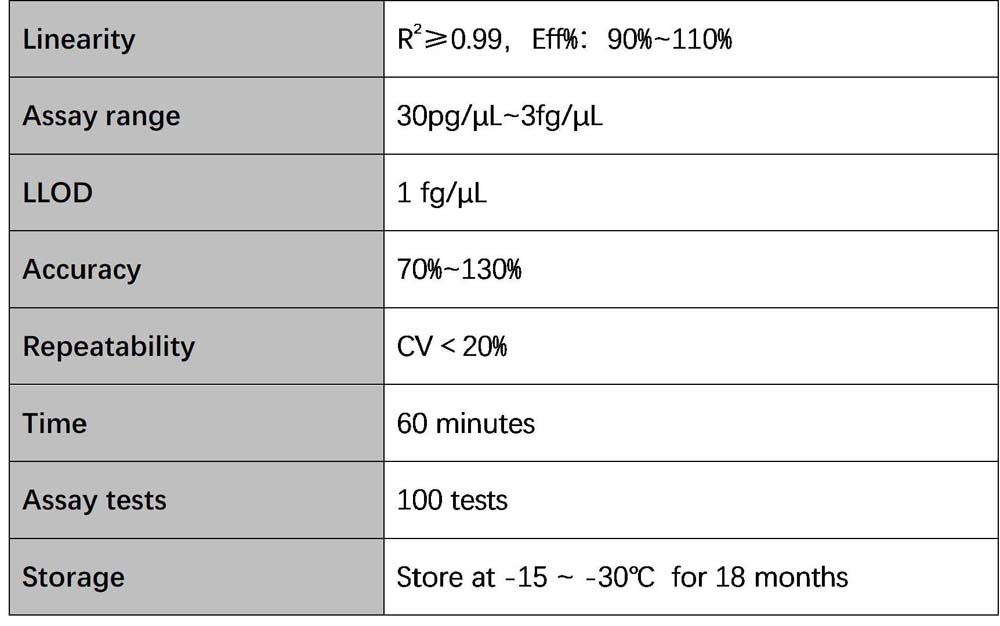
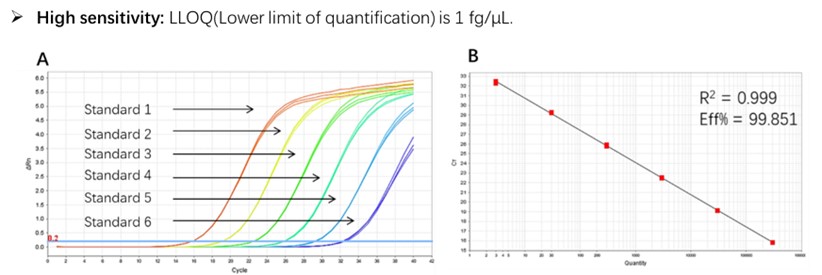
Figure 1. High sensitivity and broad dynamic range using the E. coli residual DNA quantitative Kit. (A) Typical analysis results obtained with Standard 1 (300 pg/μL) to 6 (3 fg/μL). (B) The standard curve of the 10-fold dilution series. PCR efficiency should be 90-110%.
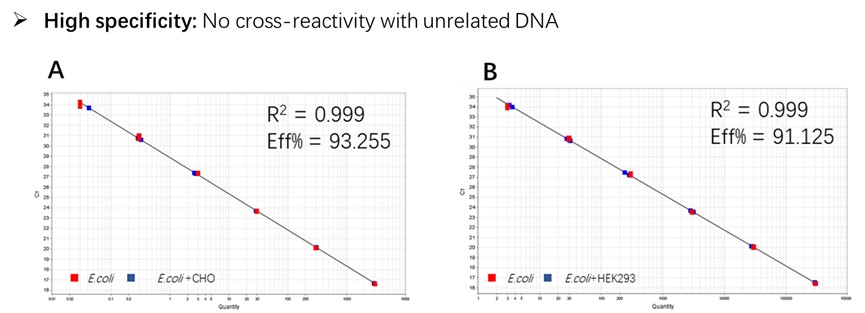
Figure 2. Assay specificity. Standard curves generated using 10-fold serial dilution of E. coli genomic DNA (included in the kit).
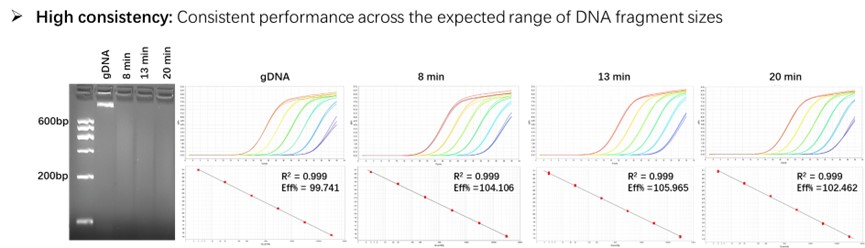
Figure 3. Consistent quantitation across a broad range of fragment sizes. Standard curves were generated using a 10-fold serial dilution of gDNA and fragmented DNA. Fragmented DNA was generated by sonicating total E. coli genomic DNA (8min, 13min, 20min). Fragmentation of the DNA was confirmed by agarose gel analysis.
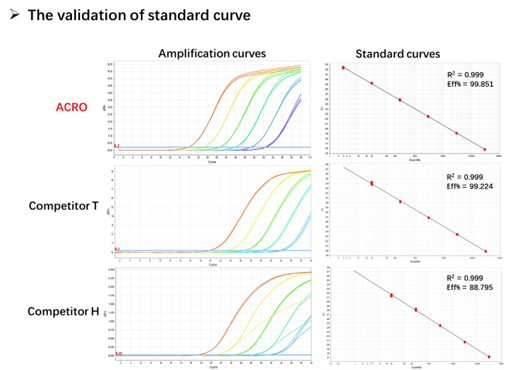
Figure 4. The amplification efficiency of standard curve (ACRO and Competitor T) is close to 100%, while the amplification efficiency of standard curve (Competitor H) is less than 90%.
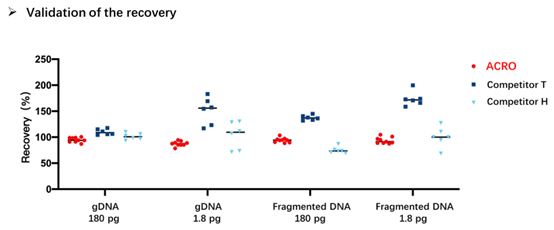
Figure 5. Validation of the recovery in four different E. coli DNA samples (gDNA 180 pg, gDNA 1.8 pg, Fragmented DNA 180 pg, Fragmented DNA 1.8 pg). Results show that the recovery using the ACRO products are between 90 and 100%.
| ID | Components | Size |
| OPA-O002-01 | 2×qPCR Master Mix | 1.0 mL×2 |
| OPA-O002-02 | E. coli Primer & Probe Mix | 700 μL |
| OPA-O002-03 | E. coli DNA Control(3ng/μL) | 100 μL |
| OPA-O002-04 | Dilution Buffer | 1.5 mL×3 |
| OPA-O002-05 | DNase/RNase-free Water | 1.0 mL |

ACROBiosystems는 생체 활성이 높은 균질한 CD3 δ/CD3 ε 및 CD3 γ/CD3 ε 단백질을 개발하여 이중특이 항체를 치료제로의 임상 개발을 가속시킵니다.

We offer a wide range of cell and gene therapy solutions starting from discovery to the clinic. Explore our wide range of proteins, antibodies, kits, and other assays to accelerate the development of your cell and gene therapy.

오가노이드 툴 박스는 약물 개발 프로젝트의 진행을 가속할 수 있는 레디메이드 오가노이드와 오가노이드 분화 툴 키트 및 다양한 서비스를 포함하는 오가노이드 솔루션 모음입니다.

고품질 GMP 등급 사이토카인 IL-15,IL-7, IL-21 등 제품은 신속한 임상/신약승인신청을 위해 개발된 것입니다.

50+ car-t의 인기 타겟을 커버하고, car 발현 검사를 위해 특별히 설계된 항원 단백질은 스트림 검증을 통해 고특이성 car 발현을 검출할 수 있으며, 고 배치간 일관성을 가지고 있다.pe/fi표기 단백질로 한 단계 염색하면 고특이성, 무 백그라운드로 car 발현 검사를 검사할 수 있습니다.

cd20, claudin18.2, cd133, gprc5d, ccr5, ccr8을 포함한 안정적이고 활성이 높은 전장 다중 막관통 단백질은 면역, elisa, spr, bli, 세포 실험, car 양성률 검사 등에 광범위하게 사용된다. vlp, 스케일제거제, nanodisc의 다양한 기술 플랫폼은 다중 막관통 단백질을 목표로 하는 약물 연구에 도움을 준다.

GMP 등급 사이토카인. 고품질 세포 활성화 및 확장 시약. 유전자 편집 시약, 효소 CAR 검사 시약 등 제품. 세포 및 유전자 요법 고객님에게 포괄적인 솔루션을 제공하고 초기 약물 발견에서 임상 연구에 이르기까지 모든 단계에서 귀하와 동반하겠습니다.

현재 이미 알려진 거의 대부분의 면역 체크 포인트 분자를 포함하여 다양한 레벨과 물종을 제공하여 고객이 선택할수 있도록 하고있다. 천연중합체 형식은 mals를 통해 검증되었고 생물활성은 elisa/spr/bli/facs 등을 통해 검증되었으며 또한 biotin/fitc 표지는 높은 처리량을 가진 항체를 선별하는데 편리하다.

ACROBiosystems는 ADC약물의 연구개발을 돕기 위해 노력하고 있으며, 다양한 인기 타겟에 서로 다른 종, 다른 라벨의 제품을 개발했으며, 높은 순도, 높은 친화력 등 특징을 가지고 있으며, 면역, 항체 선택, spr, 세포 활성 검사 및 기타 실험에 적합하고, 해당 protocol을 무료로 제공합니다.

Fc 수용체 단백질은 모든 분자를 포함하고 있을 뿐만 아니라, 일반적인 돌연변이와 바이오틴 표기 종류을 포함하고 있어 고객들이 단일 클론 항체 개발을 가속화할 수 있다.

포괄적으로 풍부한 사이토카인 표적 단백질은 HEK293 진핵생물 시스템에 의해 발현되며 천연 구조에 더 가깝다. 고순도는 SDS-PAGE/HPLC/MALS에 의해 검증된다. 높은 생물학적 활성이 ELISA/SPR/BLI에 의해 검증된다. 배치간 일관성이 우수하여 항체 면역, 스크리닝 및 품질 관리 과정에 적합하다.

Aneuro는 ACROBiosystems가 뇌과학 연구에 초점을 맞춘 제품 라인으로서 치료 및 진단 연구 단백질, PFFs와 재조합 신경 인자 등 뇌과학 연구 분야에 중요한 단백질 제품을 제공하여 뇌과학 연구에 도움이 된다.
This web search service is supported by Google Inc.
