



























 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!
 Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!  Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!
Human
Purified Human CD3ε Protein.
Unconjugated
The Isotype control is sold separately and you can search for Cat. No. DNP-M1A1 for product information.
Lyophilized from 0.22 μm filtered solution in PBS, pH7.4 with trehalose as protectant.
Contact us for customized product form or formulation.
Please see Certificate of Analysis for specific instructions.
For best performance, we strongly recommend you to follow the reconstitution protocol provided in the CoA.
For long term storage, the product should be stored at lyophilized state at -20°C or lower.
Please avoid repeated freeze-thaw cycles.
This product is stable after storage at:
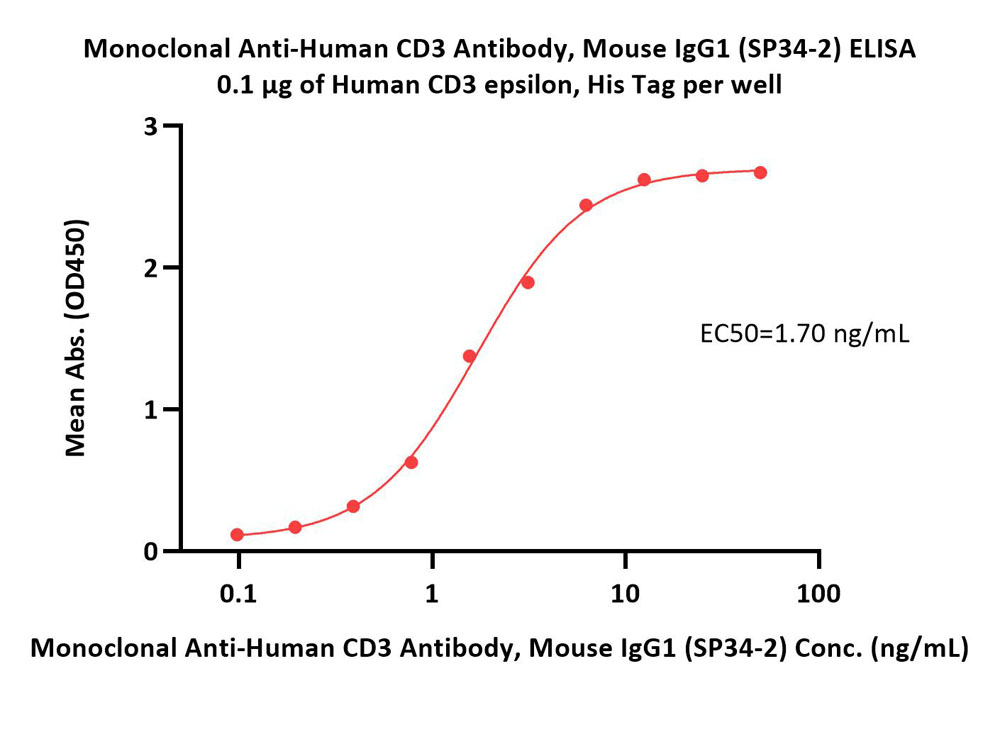
Immobilized Human CD3 epsilon, His Tag (Cat. No. CDE-H5223) at 1 μg/mL (100 μL/well) can bind Monoclonal Anti-Human CD3 Antibody, Mouse IgG1 (SP34-2) (Cat. No. CDE-M531) with a linear range of 0.4-6 ng/mL (QC tested).

2e5 of Jurkat cells were stained with 100 μL of 10 μg/mL of Monoclonal Anti-Human CD3 Antibody, Mouse IgG1 (SP34-2) (Cat. No. CDE-M531) and isotype control antibody respectively, washed and then followed by PE-anti mouse IgG1 antibody and analyzed with FACS (Routinely tested).

ACROBiosystems는 생체 활성이 높은 균질한 CD3 δ/CD3 ε 및 CD3 γ/CD3 ε 단백질을 개발하여 이중특이 항체를 치료제로의 임상 개발을 가속시킵니다.

We offer a wide range of cell and gene therapy solutions starting from discovery to the clinic. Explore our wide range of proteins, antibodies, kits, and other assays to accelerate the development of your cell and gene therapy.

오가노이드 툴 박스는 약물 개발 프로젝트의 진행을 가속할 수 있는 레디메이드 오가노이드와 오가노이드 분화 툴 키트 및 다양한 서비스를 포함하는 오가노이드 솔루션 모음입니다.

고품질 GMP 등급 사이토카인 IL-15,IL-7, IL-21 등 제품은 신속한 임상/신약승인신청을 위해 개발된 것입니다.

50+ car-t의 인기 타겟을 커버하고, car 발현 검사를 위해 특별히 설계된 항원 단백질은 스트림 검증을 통해 고특이성 car 발현을 검출할 수 있으며, 고 배치간 일관성을 가지고 있다.pe/fi표기 단백질로 한 단계 염색하면 고특이성, 무 백그라운드로 car 발현 검사를 검사할 수 있습니다.

cd20, claudin18.2, cd133, gprc5d, ccr5, ccr8을 포함한 안정적이고 활성이 높은 전장 다중 막관통 단백질은 면역, elisa, spr, bli, 세포 실험, car 양성률 검사 등에 광범위하게 사용된다. vlp, 스케일제거제, nanodisc의 다양한 기술 플랫폼은 다중 막관통 단백질을 목표로 하는 약물 연구에 도움을 준다.

GMP 등급 사이토카인. 고품질 세포 활성화 및 확장 시약. 유전자 편집 시약, 효소 CAR 검사 시약 등 제품. 세포 및 유전자 요법 고객님에게 포괄적인 솔루션을 제공하고 초기 약물 발견에서 임상 연구에 이르기까지 모든 단계에서 귀하와 동반하겠습니다.

현재 이미 알려진 거의 대부분의 면역 체크 포인트 분자를 포함하여 다양한 레벨과 물종을 제공하여 고객이 선택할수 있도록 하고있다. 천연중합체 형식은 mals를 통해 검증되었고 생물활성은 elisa/spr/bli/facs 등을 통해 검증되었으며 또한 biotin/fitc 표지는 높은 처리량을 가진 항체를 선별하는데 편리하다.

ACROBiosystems는 ADC약물의 연구개발을 돕기 위해 노력하고 있으며, 다양한 인기 타겟에 서로 다른 종, 다른 라벨의 제품을 개발했으며, 높은 순도, 높은 친화력 등 특징을 가지고 있으며, 면역, 항체 선택, spr, 세포 활성 검사 및 기타 실험에 적합하고, 해당 protocol을 무료로 제공합니다.

Fc 수용체 단백질은 모든 분자를 포함하고 있을 뿐만 아니라, 일반적인 돌연변이와 바이오틴 표기 종류을 포함하고 있어 고객들이 단일 클론 항체 개발을 가속화할 수 있다.

포괄적으로 풍부한 사이토카인 표적 단백질은 HEK293 진핵생물 시스템에 의해 발현되며 천연 구조에 더 가깝다. 고순도는 SDS-PAGE/HPLC/MALS에 의해 검증된다. 높은 생물학적 활성이 ELISA/SPR/BLI에 의해 검증된다. 배치간 일관성이 우수하여 항체 면역, 스크리닝 및 품질 관리 과정에 적합하다.

Aneuro는 ACROBiosystems가 뇌과학 연구에 초점을 맞춘 제품 라인으로서 치료 및 진단 연구 단백질, PFFs와 재조합 신경 인자 등 뇌과학 연구 분야에 중요한 단백질 제품을 제공하여 뇌과학 연구에 도움이 된다.
| English Name | Research Code | Research Phase | Company | First Brand Name | First Approved Country | First Indication | First Approved Company | First Approved Date | Indications | Clinical Trials |
|---|---|---|---|---|---|---|---|---|---|---|
| Mouse monoclonal antibody against human CD3 antigen of T lymphocyte (Wuhan Institute of Biological Products) | Approved | Wuhan Institute Of Biological Products Co Ltd | Mainland China | Rejection of organ transplantation | Wuhan Institute Of Biological Products Co Ltd | 1999-01-01 | Rejection of organ transplantation | Details | ||
| Tebentafusp | ImmTAC-gp-100; IMC-gp-100; IMC-gp100 | Approved | Immunocore Ltd | KIMMTRAK | United States | Uveal melanoma | Immunocore Ltd | 2022-01-25 | Uveal melanoma; Melanoma | Details |
| Teclistamab | JNJ-64007957; JNJ-64007959; JNJ-7957 | Approved | Johnson & Johnson Innovative Medicine, Genmab A/S | TECVAYLI, TECAYLI | EU | Multiple Myeloma | Janssen-Cilag International Nv | 2022-08-23 | Hematologic Diseases; Hematologic Neoplasms; Multiple Myeloma | Details |
| Teplizumab | MGA-031; PRV-031; hOKT3-γ1-ala-ala; hOKT3-gamma-1-ala-ala | Approved | Tolerance Therapeutics Inc | TZIELD | United States | Diabetes Mellitus, Type 1 | Provention Bio Inc | 2022-11-17 | Diabetes Mellitus, Type 1; Rejection of renal transplantation; Glucose Intolerance; Hypoglycemia; Psoriasis; Diabetes Mellitus, Experimental | Details |
| Mosunetuzumab | BTCT-4465A; RO-7030816; CD20-TBD; RG-7828 | Approved | Genentech Inc | Lunsumio | EU | Lymphoma, Follicular | Roche Registration Gmbh | 2022-06-03 | Lymphoma, B-Cell; Lymphoma, B-Cell, Marginal Zone; Lymphoma, Large B-Cell, Diffuse; Precursor B-Cell Lymphoblastic Leukemia-Lymphoma; Lymphoma, Follicular; Lupus Erythematosus, Systemic; Lymphoma; Lymphoma, Non-Hodgkin; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
| Epcoritamab | GEN-3013; ABBV-GMAB-3013 | Approved | Genmab A/S, Abbvie Inc | Tepkinly, TEPKINLY, EPKINLY | United States | Lymphoma, Large B-Cell, Diffuse | Genmab Us Inc | 2023-05-19 | Lymphoma, B-Cell; Lymphoma, B-Cell, Marginal Zone; Lymphoma, Large B-Cell, Diffuse; Lymphoma, Follicular; Lymphoma, Mantle-Cell; Primary mediastinal B cell lymphoma; Richter's Syndrome; Lymphoma; Lymphoma, Non-Hodgkin; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
| Blinatumomab | BiTE-MT-103; bscCD19xCD3; AMG-103; MT-103; MEDI-538 | Approved | Micromet Inc | Blincyto, 倍利妥 | United States | Precursor B-Cell Lymphoblastic Leukemia-Lymphoma | Amgen Inc | 2014-12-03 | Leukemia; Leukemia, Myelogenous, Chronic; Neoplasm, Residual; Lymphoma, B-Cell; Lymphoma, Large B-Cell, Diffuse; Multiple Myeloma; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Precursor B-Cell Lymphoblastic Leukemia-Lymphoma; Precursor T-Cell Lymphoblastic Leukemia-Lymphoma; Leukemia, B-Cell; Philadelphia Chromosome; Lymphoma, Non-Hodgkin; Burkitt Lymphoma; Lymphoma; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
| Mouse monoclonal antibody against human CD3 antigen of T lymphocyte (Wuhan Institute of Biological Products) | Approved | Wuhan Institute Of Biological Products Co Ltd | Mainland China | Rejection of organ transplantation | Wuhan Institute Of Biological Products Co Ltd | 1999-01-01 | Rejection of organ transplantation | Details | ||
| Tebentafusp | ImmTAC-gp-100; IMC-gp-100; IMC-gp100 | Approved | Immunocore Ltd | KIMMTRAK | United States | Uveal melanoma | Immunocore Ltd | 2022-01-25 | Uveal melanoma; Melanoma | Details |
| Teclistamab | JNJ-64007957; JNJ-64007959; JNJ-7957 | Approved | Johnson & Johnson Innovative Medicine, Genmab A/S | TECVAYLI, TECAYLI | EU | Multiple Myeloma | Janssen-Cilag International Nv | 2022-08-23 | Hematologic Diseases; Hematologic Neoplasms; Multiple Myeloma | Details |
| Teplizumab | MGA-031; PRV-031; hOKT3-γ1-ala-ala; hOKT3-gamma-1-ala-ala | Approved | Tolerance Therapeutics Inc | TZIELD | United States | Diabetes Mellitus, Type 1 | Provention Bio Inc | 2022-11-17 | Diabetes Mellitus, Type 1; Rejection of renal transplantation; Glucose Intolerance; Hypoglycemia; Psoriasis; Diabetes Mellitus, Experimental | Details |
| Mosunetuzumab | BTCT-4465A; RO-7030816; CD20-TBD; RG-7828 | Approved | Genentech Inc | Lunsumio | EU | Lymphoma, Follicular | Roche Registration Gmbh | 2022-06-03 | Lymphoma, B-Cell; Lymphoma, B-Cell, Marginal Zone; Lymphoma, Large B-Cell, Diffuse; Precursor B-Cell Lymphoblastic Leukemia-Lymphoma; Lymphoma, Follicular; Lupus Erythematosus, Systemic; Lymphoma; Lymphoma, Non-Hodgkin; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
| Epcoritamab | GEN-3013; ABBV-GMAB-3013 | Approved | Genmab A/S, Abbvie Inc | Tepkinly, TEPKINLY, EPKINLY | United States | Lymphoma, Large B-Cell, Diffuse | Genmab Us Inc | 2023-05-19 | Lymphoma, B-Cell; Lymphoma, B-Cell, Marginal Zone; Lymphoma, Large B-Cell, Diffuse; Lymphoma, Follicular; Lymphoma, Mantle-Cell; Primary mediastinal B cell lymphoma; Richter's Syndrome; Lymphoma; Lymphoma, Non-Hodgkin; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
| Blinatumomab | BiTE-MT-103; bscCD19xCD3; AMG-103; MT-103; MEDI-538 | Approved | Micromet Inc | Blincyto, 倍利妥 | United States | Precursor B-Cell Lymphoblastic Leukemia-Lymphoma | Amgen Inc | 2014-12-03 | Leukemia; Leukemia, Myelogenous, Chronic; Neoplasm, Residual; Lymphoma, B-Cell; Lymphoma, Large B-Cell, Diffuse; Multiple Myeloma; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Precursor B-Cell Lymphoblastic Leukemia-Lymphoma; Precursor T-Cell Lymphoblastic Leukemia-Lymphoma; Leukemia, B-Cell; Philadelphia Chromosome; Lymphoma, Non-Hodgkin; Burkitt Lymphoma; Lymphoma; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
| English Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| M-701 | M-701 | Phase 3 Clinical | Wuhan Yzy Biopharma Co Ltd | Ovarian Neoplasms; Solid tumours; Hydrothorax; Stomach Neoplasms; Neoplasms; Ascites; Colorectal Neoplasms; Pleural Effusion, Malignant; Carcinoma, Non-Small-Cell Lung | Details |
| ABBV-383 | TNB-383B; ABBV-383 | Phase 3 Clinical | Teneobio Inc, Abbvie Inc | Multiple Myeloma | Details |
| IMC-F106C | IMC-F106C; PRAME HLA-A02 | Phase 3 Clinical | Immunocore Ltd | Solid tumours; Melanoma | Details |
| Alnuctamab | CC-93269; EM-901; BMS-986349 | Phase 3 Clinical | Engmab Ag | Multiple Myeloma | Details |
| Catumaxomab | LP000 | Phase 3 Clinical | Trion Research, Neovii Biotech Gmbh | Ovarian Neoplasms; Stomach Neoplasms; Carcinoma; Neoplasms; Carcinoma, Ovarian Epithelial; Colonic Neoplasms; Urinary Bladder Neoplasms; Ascites; Breast Neoplasms | Details |
| Flotetuzumab | S-80880; MGD-006; RES-234 | Phase 2 Clinical | Macrogenics Inc | Leukemia, Myelogenous, Chronic; Hematologic Neoplasms; Leukemia; Leukemia, Hairy Cell; Mastocytosis, Systemic; Hodgkin Disease; Blastic Plasmacytoid Dendritic Cell Neoplasm; Precursor T-Cell Lymphoblastic Leukemia-Lymphoma; Neoplasms, Plasma Cell; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Leukemia, B-Cell; Leukemia, Myeloid, Acute; Leukemia, Biphenotypic, Acute | Details |
| Anti-CD3/humanized 3F8 bispecific antibody-activated T lymphocytes (University of Virginia) | Phase 2 Clinical | University Of Virginia | Neuroblastoma | Details | |
| Imvotamab | IGM-2323 | Phase 2 Clinical | Igm Biosciences Inc | Lymphoma, B-Cell, Marginal Zone; Myositis; Lupus Erythematosus, Cutaneous; Arthritis, Rheumatoid; Lymphoma, Large B-Cell, Diffuse; Lupus Erythematosus, Systemic; Lymphoma, Mantle-Cell; Lymphoma, Follicular; Arthritis; Lymphoma, Non-Hodgkin | Details |
| Foralumab | TZLS-401; NI-0401/α-CD3; NI-0401 | Phase 2 Clinical | Novimmune Sa, Bristol-Myers Squibb Company | Respiratory Tract Infections; Diabetes Mellitus, Type 2; Metabolic Dysfunction-Associated Steatotic Liver Disease; Coronavirus Disease 2019 (COVID-19); Multiple Sclerosis, Chronic Progressive; Multiple Sclerosis; Alzheimer Disease; Crohn Disease | Details |
| GNR-084 | GNR-084 | Phase 2 Clinical | Generium Pharmaceuticals, Iontas | Precursor Cell Lymphoblastic Leukemia-Lymphoma; Precursor B-Cell Lymphoblastic Leukemia-Lymphoma | Details |
| Anti-CD3 and anti-HER2 BiTE-expressing T cell (MedImmune) | Phase 2 Clinical | University Of Michigan, Huazhong University Of Science And Technology, University Of Virginia Cancer Center, Medimmune | Breast Neoplasms | Details | |
| Vibecotamab | XmAb-14045 | Phase 2 Clinical | Xencor Inc | Leukemia, Myeloid; Myelodysplastic Syndromes; Blastic Plasmacytoid Dendritic Cell Neoplasm; Precursor B-Cell Lymphoblastic Leukemia-Lymphoma; Leukemia, Myeloid, Acute | Details |
| α/β CD3+/CD19+ cell depleted stem cell therapy (Mitchell Cairo) | Phase 2 Clinical | New York Medical College | Leukemia; Anemia; Thalassemia; Hodgkin Disease; Anemia, Aplastic; Thrombocytopenia; Lymphoma, Non-Hodgkin; Kostmann Syndrome; Anemia, Sickle Cell | Details | |
| MK-6070 | HPN-328; MK-6070 | Phase 2 Clinical | Harpoon Therapeutics | Small Cell Lung Carcinoma | Details |
| Cibisatamab | CEA-TCB; RG-7802; RO-6958688; CEA-CD3 TCB | Phase 2 Clinical | F. Hoffmann-La Roche Ltd | Solid tumours; Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| CD3/CD19 neg allogeneic BMT (National Institute of Allergy and Infectious Diseases/University of Pittsburgh) | Phase 2 Clinical | National Institute Of Allergy And Infectious Diseases (Niaid), University Of Pittsburgh | Primary Immunodeficiency Diseases; Female Urogenital Diseases; Inflammation | Details | |
| HPN-536 | HPN-536 | Phase 2 Clinical | Harpoon Therapeutics | Neoplasms | Details |
| MBS-303 | MBS-303 | Phase 2 Clinical | Beijing Mabworks Biotech Co Ltd | Lymphoma, B-Cell; Lymphoma, Non-Hodgkin | Details |
| HBM-7022 | HBM-7022; AZD-5863 | Phase 2 Clinical | Harbour Biomed | Esophageal Neoplasms; Stomach Neoplasms; Pancreatic Neoplasms; Neoplasms; Digestive System Neoplasms; Esophageal adenocarcinoma; Carcinoma, Pancreatic Ductal; Gastrointestinal Neoplasms | Details |
| TAK-280 | MVC-280; TAK-280 | Phase 2 Clinical | Maverick Therapeutics Inc | Solid tumours; Neoplasms; Neoplasm Metastasis | Details |
| GB-261 | GB-261 | Phase 2 Clinical | Genor Biopharma Co Ltd | Lymphoma, B-Cell; Leukemia, Lymphoid; Lymphoma, Non-Hodgkin; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
| REGN-5459 | REGN-5459 | Phase 2 Clinical | Multiple Myeloma; Renal Insufficiency, Chronic | Details | |
| IBI-3003 | IBI3003; IBI-3003 | Phase 2 Clinical | Innovent Biologics(Suzhou) Co Ltd | Multiple Myeloma | Details |
| MBS-314 | MBS-314 | Phase 2 Clinical | Beijing Mabworks Biotech Co Ltd | Multiple Myeloma | Details |
| RO7515629 | RO7515629; RO-7515629; RG-6353 | Phase 2 Clinical | F. Hoffmann-La Roche Ag | Ovarian Neoplasms; Carcinoma, Renal Cell; Pancreatic Neoplasms; Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| BI-764532 | BI-764532; OBT620 | Phase 2 Clinical | C.H. Boehringer Sohn Ag & Co. Kg | Small Cell Lung Carcinoma; Neoplasms; Neuroendocrine Tumors; Carcinoma, Neuroendocrine; Glioma | Details |
| GEN3017 | GEN-3017 | Phase 2 Clinical | Genmab A/S | Hematologic Neoplasms; Hodgkin Disease; Lymphoma, Non-Hodgkin | Details |
| MP0533 | MP0533 | Phase 2 Clinical | Molecular Partners Ag | Leukemia, Myeloid, Acute | Details |
| CD30 biAb-AATC(The Medical College Of Wisconsin Nonprofit) | Phase 2 Clinical | The Medical College Of Wisconsin Nonprofit | Lymphoma, B-Cell; Leukemia; Hodgkin Disease; Lymphoma; Lymphoma, Large-Cell, Anaplastic; Lymphoma, T-Cell, Cutaneous | Details | |
| APVO-436 | APVO-436; APVO436 | Phase 2 Clinical | Aptevo | Myelodysplastic Syndromes; Leukemia, Myeloid, Acute | Details |
| Emfizatamab | GNC-038 | Phase 2 Clinical | SystImmune | Solid tumours; Lymphoma, Large B-Cell, Diffuse; Lymphoma, Extranodal NK-T-Cell; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Lymphoma, Non-Hodgkin; Lymphoma, T-Cell; Central Nervous System Lymphoma | Details |
| GEN-1047 | GEN-1047 | Phase 2 Clinical | Genmab A/S | Carcinoma, Non-Small-Cell Lung | Details |
| REGN-4336 | REGN-4336 | Phase 2 Clinical | Prostatic Neoplasms, Castration-Resistant | Details | |
| CM-350 | CM-350 | Phase 2 Clinical | Keymed Biosciences Co Ltd | Solid tumours | Details |
| 1-A-46 | BR-110; 1A46; 1-A-46; CMG1A46; CMG1A-46; BR110 | Phase 2 Clinical | Chengdu Chimagen Biosciences Co Ltd, BioRay Pharmaceutical Co Ltd | Lymphoma, B-Cell; Neoplasms; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Lymphoma, Non-Hodgkin | Details |
| LBL-034 | LBL-034 | Phase 2 Clinical | Nanjing Leads Biolabs Co Ltd | Neoplasms; Multiple Myeloma | Details |
| LBL-033 | LBL-033 | Phase 2 Clinical | Nanjing Leads Biolabs Co Ltd | Neoplasms | Details |
| CM-336(Connaught Biomedical Technology) | CM-336 | Phase 2 Clinical | Keymed Biosciences Co Ltd | Multiple Myeloma | Details |
| ICP-B02 | CM-355; ICP-B02 | Phase 2 Clinical | Beijing Tiannuo Jiancheng Pharmaceutical Technology Co Ltd, Keymed Biosciences Co Ltd | Hematologic Neoplasms; Hematoma; Lymphoma; Lymphoma, Non-Hodgkin | Details |
| SMET-12 | SMET-12 | Phase 2 Clinical | Zhejiang Shimai Pharmaceutical Co Ltd | Solid tumours; Carcinoma, Non-Small-Cell Lung | Details |
| QLS-31905 | QLS-31905 | Phase 2 Clinical | Qilu Pharmaceutical Co Ltd | Solid tumours; Neoplasms | Details |
| Nebratamig | GNC-035 | Phase 2 Clinical | Solid tumours; Hematologic Neoplasms; Breast Neoplasms; Metastatic breast cancer; Lymphoma, Non-Hodgkin; Leukemia, Lymphocytic, Chronic, B-Cell | Details | |
| TAK-186 | EGFR x CD3 COBRA; MVC-101; TAK-186 | Phase 2 Clinical | Takeda Pharmaceutical Co Ltd | Solid tumours; Squamous Cell Carcinoma of Head and Neck; Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| EMB-06 | EMB-06; EMB06 | Phase 2 Clinical | Shanghai Epimab Biotherapeutics, Inc | Multiple Myeloma | Details |
| AZD-0486 | TNB-486; AZD-0486; AZD0486 | Phase 2 Clinical | Teneobio Inc | Lymphoma, B-Cell; Lymphoma, Large B-Cell, Diffuse; Lymphoma, Follicular; Precursor B-Cell Lymphoblastic Leukemia-Lymphoma; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Leukemia, Myeloid, Acute; Lymphoma, Non-Hodgkin | Details |
| BNT-142 | BNT-142 | Phase 2 Clinical | Biontech Se | Solid tumours | Details |
| CN-201 | CN-201 | Phase 2 Clinical | Curon Biopharmaceutical Ltd | Lymphoma, B-Cell; Precursor B-Cell Lymphoblastic Leukemia-Lymphoma; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Leukemia, B-Cell; Lymphoma, Non-Hodgkin | Details |
| ZG006 | ZG006; ZG-006 | Phase 2 Clinical | Gensun Biopharma Inc, Suzhou Zelgen Biopharmaceuticals Co Ltd | Solid tumours; Neoplasms; Small Cell Lung Carcinoma; Carcinoma, Neuroendocrine | Details |
| EX-103 | EX-103; EX103 | Phase 2 Clinical | Guangzhou Excelmab Inc | Arthritis, Rheumatoid; Lymphoma, Non-Hodgkin; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
| Anti-CD3 monoclonal antibody (Jinan Tiankang Biological Products) | Phase 2 Clinical | Jinan Tiankang Biological Products Co Ltd | Anemia, Aplastic | Details | |
| Cevostamab | RG-6160; BFCR-4350A; RO-7187797 | Phase 2 Clinical | Genentech Inc | Multiple Myeloma | Details |
| Anti-CD3/anti-EGFR -activated T cells (Barbara Ann Karmanos Cancer Institute) | Phase 2 Clinical | Barbara Ann Karmanos Cancer Institute, University Of Virginia | Glioblastoma; Pancreatic Neoplasms | Details | |
| Ubamatamab | REGN-4018 | Phase 2 Clinical | Ovarian Neoplasms; Peritoneal Neoplasms; Endometrial Neoplasms; Fallopian Tube Neoplasms | Details | |
| Anti-CD3-anti-HER2-activated T cells | Phase 2 Clinical | Transtarget | Prostatic Neoplasms, Castration-Resistant; Breast Neoplasms; Prostatic Neoplasms | Details | |
| JCAR-014 | JCAR-014; JCAR021 | Phase 2 Clinical | Fred Hutchinson Cancer Research Center, Memorial Sloan Kettering Cancer Center, Seattle Children'S Research Institute, Juno Therapeutics Inc | Lymphoma, B-Cell; Leukemia; Leukemia, Lymphoid; Hodgkin Disease; Lymphoma, Large B-Cell, Diffuse; Candidiasis; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Lymphoma, Mantle-Cell; Primary mediastinal B cell lymphoma; Leukemia, B-Cell; Lymphoma, Non-Hodgkin; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
| A-319 | A-319 | Phase 1 Clinical | Evive Biotech Ltd | Lymphoma, B-Cell; Precursor B-Cell Lymphoblastic Leukemia-Lymphoma; Leukemia, B-Cell | Details |
| BCMA TriTAC | HPN-217 | Phase 1 Clinical | Harpoon Therapeutics, Abbvie Inc | Multiple Myeloma | Details |
| Membrane bound ligand T-SIGn virus | NG-348 | Phase 1 Clinical | Akamis Bio Ltd | Neoplasms | Details |
| NG-641 | EnAd-FAP-BiTE; EnAd-FAP-Tac; NG-641; NG-aFAP | Phase 1 Clinical | Akamis Bio Ltd, University Of Oxford | Squamous Cell Carcinoma of Head and Neck; Neoplasms, Glandular and Epithelial; Neoplasm Metastasis | Details |
| Vixtimotamab | T-564; AMV-564 | Phase 1 Clinical | Amphivena Therapeutics Inc | Solid tumours; Myelodysplastic Syndromes | Details |
| MGD-014 | MGD-014 | Phase 1 Clinical | Macrogenics Inc | HIV Infections | Details |
| JNJ-63898081 | JNJ-8081; JNJ-63898081 | Phase 1 Clinical | Johnson & Johnson Innovative Medicine | Neoplasms | Details |
| K-193 | K-193 | Phase 1 Clinical | Beijing Lvzhu Biological Technology Co Ltd | Lymphoma, B-Cell | Details |
| Anti-EGFR-bispecific antibody armed activated T-cell therapy (Memorial Sloan Kettering Cancer Center) | Phase 1 Clinical | Memorial Sloan Kettering Cancer Center | Pancreatic Neoplasms | Details | |
| Runimotamab | BTRC-4017A; RG-6194; RO-7227780 | Phase 1 Clinical | F. Hoffmann-La Roche Ltd | Solid tumours; Neoplasms | Details |
| Autologous T-cell therapy (anti-PSMA-CD3), Roger Williams Medical Center | Phase 1 Clinical | Roger Williams Medical Center | Prostatic Neoplasms | Details | |
| ONO-4685 | ONO-4685 | Phase 1 Clinical | Merus Nv | Psoriasis; Lymphoma, T-Cell; Plaque psoriasis | Details |
| WVT-078(Novartis Pharma) | WVT-078 | Phase 1 Clinical | Novartis Pharma Ag | Multiple Myeloma | Details |
| M-802(Wuhan Yzy Biopharma/CSPC Pharmaceutical) | M-802 | Phase 1 Clinical | Wuhan Yzy Biopharma Co Ltd, CSPC Pharmaceutical Group Ltd | Ovarian Neoplasms; Solid tumours; Stomach Neoplasms; Breast Neoplasms | Details |
| Emirodatamab | AMG-427 | Phase 1 Clinical | Amgen Inc | Leukemia, Myeloid, Acute | Details |
| JNJ-63709178 | JNJ-9178; CNTO-9958; JNJ-63709178 | Phase 1 Clinical | Johnson & Johnson Innovative Medicine, Genmab A/S | Leukemia, Myeloid, Acute | Details |
| SAR-442257 | SAR-442257 | Phase 1 Clinical | Sanofi | Neoplasms | Details |
| CC-1 | CC-1 | Phase 1 Clinical | University Of Tubingen, German Cancer Research Center (Dkfz) | Prostatic Neoplasms, Castration-Resistant; Prostatic Neoplasms; Carcinoma, Squamous Cell | Details |
| Acapatamab | AMG-160 | Phase 1 Clinical | Amgen Inc | Prostatic Neoplasms, Castration-Resistant; Carcinoma, Non-Small-Cell Lung | Details |
| Pavurutamab | AMG-701 | Phase 1 Clinical | Amgen Inc | Multiple Myeloma | Details |
| Xaluritamig | AMG-509 | Phase 1 Clinical | Xencor Inc, Amgen Inc | Prostatic Neoplasms, Castration-Resistant | Details |
| CC-3 | CC-3 | Phase 1 Clinical | Eberhard Karls University Of Tubingen, Germany | Colorectal Neoplasms; Gastrointestinal Neoplasms | Details |
| JS-203 | JS-203 | Phase 1 Clinical | Shanghai Junshi Biological Engineering Co Ltd | Lymphoma, B-Cell; Lymphoma, Non-Hodgkin | Details |
| BC-004 | BC-004 | Phase 1 Clinical | Shandong Buchang Pharmaceuticals Co Ltd | Solid tumours; Breast Neoplasms | Details |
| JY-016 | JY-016 | Phase 1 Clinical | Beijing Jingyitaixiang Technology Development Co Ltd, Beijing Eastern Biotech Co Ltd | Solid tumours; Colorectal Neoplasms; Lung Neoplasms | Details |
| EX-105 | EX-105; EX105 | Phase 1 Clinical | Guangzhou Excelmab Inc | Solid tumours | Details |
| EMB-07 | EMB-07 | Phase 1 Clinical | Shanghai Epimab Biotherapeutics, Inc | Solid tumours; Ovarian Neoplasms; Stomach Neoplasms; Neoplasms; Triple Negative Breast Neoplasms; Adenocarcinoma of Lung; Pancreatic Neoplasms; Urinary Bladder Neoplasms; Prostatic Neoplasms; Colorectal Neoplasms; Lymphoma; Uterine Neoplasms; Neoplasm Metastasis | Details |
| BA-3182 | BA-3182 | Phase 1 Clinical | Bioatla | Solid tumours; Adenocarcinoma | Details |
| BA-1202 | BA-1202 | Phase 1 Clinical | Solid tumours | Details | |
| CLN-978 | CLN-978 | Phase 1 Clinical | Adimab LLC | Precursor B-Cell Lymphoblastic Leukemia-Lymphoma; Lymphoma, Non-Hodgkin | Details |
| MGD-024 | MGD-024 | Phase 1 Clinical | Macrogenics Inc | Leukemia, Myelogenous, Chronic; Leukemia, Hairy Cell; Hodgkin Disease; Myelodysplastic Syndromes; Neoplasms; Blastic Plasmacytoid Dendritic Cell Neoplasm; Leukemia, B-Cell; Leukemia, Myeloid, Acute | Details |
| ARB-202 | ARB-202 | Phase 1 Clinical | Arbele Corp | Liver Neoplasms; Biliary Tract Neoplasms; Stomach Neoplasms; Pancreatic Neoplasms; Cholangiocarcinoma; Colorectal Neoplasms; Esophageal adenocarcinoma; Gastrointestinal Neoplasms | Details |
| CX-904 | CX-904 | Phase 1 Clinical | Amgen Inc, Cytomx Therapeutics Inc | Solid tumours; Neoplasms | Details |
| CLN-049 | CLN-049 | Phase 1 Clinical | University Of Tubingen, German Cancer Research Center (Dkfz) | Myelodysplastic Syndromes; Leukemia, Myeloid, Acute | Details |
| XmAb-819 | XmAb-819 | Phase 1 Clinical | Xencor Inc | Kidney Neoplasms; Carcinoma, Renal Cell | Details |
| JNJ-70218902 | JNJ-70218902 | Phase 1 Clinical | Johnson & Johnson Innovative Medicine | Neoplasms | Details |
| JNJ-67571244 | JNJ-67571244; JNJ-67371244; JNJ-1244 | Phase 1 Clinical | Johnson & Johnson | Myelodysplastic Syndromes; Leukemia, Myeloid, Acute | Details |
| SCTB-35 | SCTB35; SCTB-35 | Phase 1 Clinical | SinoCelltech Ltd | Lymphoma, B-Cell | Details |
| JNJ-87890387 | JNJ-87890387 | Phase 1 Clinical | Janssen Research & Development Llc | Solid tumours | Details |
| XmAb-541 | XmAb541; XmAb-541 | Phase 1 Clinical | Xencor Inc | Ovarian Neoplasms; Ovarian germ cell tumor; Germinoma; Endometrial Neoplasms; Neoplasms, Germ Cell and Embryonal | Details |
| [89Zr]Zr-BI-764532 | Phase 1 Clinical | C.H. Boehringer Sohn Ag & Co. Kg, Boehringer Ingelheim Gmbh | Small Cell Lung Carcinoma; Carcinoma, Neuroendocrine | Details | |
| TGI-6 | TGI-6 | Phase 1 Clinical | Hefei TG ImmunoPharma Co Ltd | Solid tumours; Colorectal Neoplasms | Details |
| SIM-0500 | SIM-0500; SIM0500 | Phase 1 Clinical | Hainan Xiansheng Re Ming Pharmaceutical Co Ltd | Bone Marrow Neoplasms; Multiple Myeloma | Details |
| Recombinant anti BCMA/CD3 bispecific antibody(Hualan Genetic Engineering) | Phase 1 Clinical | Hualan Genetic Engineering (Henan) Co Ltd | Multiple Myeloma | Details | |
| Oncolytic Virus R130(Yunying Medical) | R-130-OV; R130; R-130 | Phase 1 Clinical | Shanghai Yunying Medical Technology Co Ltd | Breast Neoplasms; Carcinoma, Non-Small-Cell Lung; Gastrointestinal Neoplasms; Melanoma; Laryngeal Neoplasms; Pharyngeal Neoplasms; Lung Neoplasms; Fallopian Tube Neoplasms; Colorectal Neoplasms; Genital Neoplasms, Female; Bone Neoplasms; Peritoneal Neoplasms; Sarcoma; Osteosarcoma; Head and Neck Neoplasms; Brain Neoplasms; Otorhinolaryngologic Neoplasms; Small Cell Lung Carcinoma; Pancreatic Neoplasms; Nose Neoplasms; Stomach Neoplasms; Esophageal Neoplasms; Carcinoma; Carcinoma, Bronchogenic; Liver Neoplasms; Ovarian Neoplasms; Kidney Neoplasms | Details |
| ASP2074 | ASP-2074; ASP2074 | Phase 1 Clinical | Astellas Pharma Global Development Inc | Solid tumours | Details |
| OBT620 | OBT620 | Phase 1 Clinical | Boehringer Ingelheim Gmbh, Oxford Biotherapeutics Ltd | Small Cell Lung Carcinoma | Details |
| CBA-1535 | CBA-1535 | Phase 1 Clinical | Chiome Bioscience Inc | Small Cell Lung Carcinoma; Triple Negative Breast Neoplasms; Mesothelioma; Carcinoma, Non-Small-Cell Lung | Details |
| JANX-008 | EGFR-TRACTr; JANX008; JANX-008 | Phase 1 Clinical | Janux Therapeutics Inc | Solid tumours; Squamous Cell Carcinoma of Head and Neck; Carcinoma, Renal Cell; Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| JANX-007 | JANX-007; PSMA-TRACTr | Phase 1 Clinical | Janux Therapeutics Inc | Prostatic Neoplasms, Castration-Resistant; Prostatic Neoplasms | Details |
| TQB-2934 | TQB2934; TQB-2934 | Phase 1 Clinical | Nanjing Shunxin Pharmaceuticals Co Ltd Of Chiatai Tianqing Pharmaceutical Group | Multiple Myeloma | Details |
| JNJ-80948543 | JNJ-80948543 | Phase 1 Clinical | Janssen Research & Development Llc | Lymphoma, B-Cell; Neoplasms; Lymphoma, Non-Hodgkin; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
| PIT-565 | PIT-565 | Phase 1 Clinical | Novartis Pharma Ag | Lymphoma, B-Cell; Precursor B-Cell Lymphoblastic Leukemia-Lymphoma; Lupus Erythematosus, Systemic; Leukemia, Myeloid, Acute | Details |
| CC-312 | CC-312 | Phase 1 Clinical | CytoCares (Shanghai) Inc | Hematologic Neoplasms; Lymphoma, B-Cell; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Lymphoma, Non-Hodgkin; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
| ASP-2138 | ASP-2138 | Phase 1 Clinical | Astellas Pharma Global Development Inc, Xencor Inc | Stomach Neoplasms; Esophageal Neoplasms; Pancreatic Neoplasms | Details |
| ISB-2001 | ISB-2001 | Phase 1 Clinical | Ichnos Sciences Sa | Multiple Myeloma | Details |
| GR-1901 | GR1901; GR-1901 | Phase 1 Clinical | Chongqing Zhixiang Jintai Biopharmaceutical Co Ltd, Genrix (Shanghai) Biopharmaceutical Co Ltd | Leukemia, Myeloid, Acute | Details |
| AMG-794 | AMG-794 | Phase 1 Clinical | Amgen Inc | Carcinoma, Ovarian Epithelial; Carcinoma, Non-Small-Cell Lung | Details |
| QLS-31904 | QLS31904; QLS-31904 | Phase 1 Clinical | Qilu Pharmaceutical Co Ltd | Solid tumours | Details |
| YK-012 | YK012 | Phase 1 Clinical | Lymphoma, B-Cell | Details | |
| RO-7428731 | RO-7428731; RG-6156 | Phase 1 Clinical | F. Hoffmann-La Roche Ltd | Glioblastoma | Details |
| GBR-1342 | GBR-1342; ISB-1342 | Phase 1 Clinical | Glenmark Pharmaceuticals Ltd | Multiple Myeloma | Details |
| Recombinant humanized anti-CD19/CD3 bispecific antibody(New Time Pharmaceutical) | LNF-1904 | Phase 1 Clinical | Shandong New Time Pharmaceutical Co Ltd | Lymphoma, B-Cell; Leukemia, B-Cell | Details |
| XmAb-968 | XmAb968 | Phase 1 Clinical | Xencor Inc | Hematologic Neoplasms; Leukemia, Promyelocytic, Acute | Details |
| GR-1803 | GR-1803 | Phase 1 Clinical | Chongqing Zhixiang Jintai Biopharmaceutical Co Ltd, Genrix (Shanghai) Biopharmaceutical Co Ltd | Multiple Myeloma | Details |
| XmAb-18968 | XmAb-18968 | Phase 1 Clinical | The Medical College Of Wisconsin Nonprofit | Leukemia, Myeloid, Acute | Details |
| SQZ-622(Novartis Pharma) | SQZ-622 | Phase 1 Clinical | Novartis Pharma Ag | Leukemia, Myeloid, Acute | Details |
| Forimtamig | RG-6234 | Phase 1 Clinical | F. Hoffmann-La Roche Ltd | Multiple Myeloma | Details |
| RG-6232 | RG-6232 | Phase 1 Clinical | F. Hoffmann-La Roche Ltd | Melanoma | Details |
| RG-6007 | RO-7283420; RG-6007 | Phase 1 Clinical | F. Hoffmann-La Roche Ltd | Leukemia, Myeloid, Acute | Details |
| SAR-443216 | SAR-443216 | Phase 1 Clinical | Sanofi | Solid tumours; Stomach Neoplasms; Neoplasms; Breast Neoplasms; Lung Neoplasms | Details |
| Recombinant oncolytic type II herpes simplex virus (Binhui Biopharmaceutical) | BS-006; oHSV2-BiTEs | Phase 1 Clinical | Wuhan Binhui Biotechnology Co Ltd | Solid tumours; Neoplasms; Melanoma; Uterine Cervical Neoplasms | Details |
| JNJ-78306358 | JNJ-78306358; JNJ-6358 | Phase 1 Clinical | Janssen Research & Development Llc | Solid tumours | Details |
| F-182112 | F-182112; F182112 | Phase 1 Clinical | Shandong New Time Pharmaceutical Co Ltd | Multiple Myeloma | Details |
| TNB-585 | TNB-585; AMG-340 | Phase 1 Clinical | Teneobio Inc | Prostatic Neoplasms, Castration-Resistant | Details |
| BC-3448 | BC3448; BC-3448 | Phase 1 Clinical | Solid tumours | Details | |
| IBI-389 | IBI-389 | Phase 1 Clinical | Innovent Biologics(Suzhou) Co Ltd | Solid tumours; Neoplasms | Details |
| hEGFRvIII-CD3 Bi-scFv | Phase 1 Clinical | Duke University | Glioblastoma; Glioma | Details | |
| JNJ-78278343 | Phase 1 Clinical | Janssen Research & Development Llc | Prostatic Neoplasms, Castration-Resistant; Prostatic Neoplasms | Details | |
| TQB-2825 | TQB-2825 | Phase 1 Clinical | Wuxi Biologics Co Ltd | Hematologic Neoplasms | Details |
| RGV-004 | RGV-004 | Phase 1 Clinical | Hangzhou Rongu Biotechnology Co Ltd | Lymphoma, B-Cell | Details |
| Recombinant humanized anti-BCMA/CD3 bispecific antibody(New Time Pharmaceutical) | Phase 1 Clinical | Shandong New Time Pharmaceutical Co Ltd | Multiple Myeloma | Details | |
| NVG-111 | NVG-111 | Phase 1 Clinical | NovalGen Ltd | Lymphoma, Large B-Cell, Diffuse; Lymphoma, Mantle-Cell; Lymphoma, Follicular; Melanoma; Carcinoma, Non-Small-Cell Lung; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
| BI-765049 | BI-765049; OBT-624 | Phase 1 Clinical | Oxford Biotherapeutics Ltd | Solid tumours | Details |
| GNC-039 | GNC-039 | Phase 1 Clinical | Solid tumours; Hematologic Neoplasms; Glioma; Neoplasm Metastasis | Details | |
| RO-7425781 | RO-7425781 | Phase 1 Clinical | F. Hoffmann-La Roche Ltd | Multiple Myeloma | Details |
| RO-7293583 | RO-7293583 | Phase 1 Clinical | F. Hoffmann-La Roche Ltd | Skin Melanoma; Uveal melanoma; Melanoma | Details |
| Rezetamig | JNJ-8780; JNJ75348780; JNJ-75348780 | Phase 1 Clinical | Teneobio Inc | Lymphoma, B-Cell; Lymphoma, Non-Hodgkin; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
| Y-150(Wuhan Yzy Biopharma/CSPC Pharmaceutical) | Y-150; Y150 | Phase 1 Clinical | Wuhan Yzy Biopharma Co Ltd, CSPC Pharmaceutical Group Ltd | Multiple Myeloma | Details |
| EX-101 | EX-101; EX101 | Phase 1 Clinical | Guangzhou Excelmab Inc | Solid tumours | Details |
| Plamotamab | XmAb-13676 | Phase 1 Clinical | Xencor Inc | Lymphoma, B-Cell; Lymphoma, Large B-Cell, Diffuse; Lymphoma, Non-Hodgkin; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
| A-337 | A-337 | Phase 1 Clinical | Evive Biotech Ltd | Solid tumours; Neoplasms | Details |
| CCW-702 | CCW-702 | Phase 1 Clinical | The Scripps Research Institute Inc, Abbvie Inc | Prostatic Neoplasms | Details |
| Tepoditamab | MCLA-117 | Phase 1 Clinical | Pharmaceutical Research Associates, Institute Gustave-Roussy, Merus Nv, Vu University Medical Center, Lgc | Leukemia, Myeloid, Acute | Details |
| ERY-974 | ERY-974 | Phase 1 Clinical | Chugai Pharmaceutical Co Ltd | Solid tumours; Carcinoma, Hepatocellular | Details |
| InHeAb-01 | InHeAb-01; bsAB | Clinical | University Hospital Tuebingen | Neoplasms | Details |
| M-701 | M-701 | Phase 3 Clinical | Wuhan Yzy Biopharma Co Ltd | Ovarian Neoplasms; Solid tumours; Hydrothorax; Stomach Neoplasms; Neoplasms; Ascites; Colorectal Neoplasms; Pleural Effusion, Malignant; Carcinoma, Non-Small-Cell Lung | Details |
| ABBV-383 | TNB-383B; ABBV-383 | Phase 3 Clinical | Teneobio Inc, Abbvie Inc | Multiple Myeloma | Details |
| IMC-F106C | IMC-F106C; PRAME HLA-A02 | Phase 3 Clinical | Immunocore Ltd | Solid tumours; Melanoma | Details |
| Alnuctamab | CC-93269; EM-901; BMS-986349 | Phase 3 Clinical | Engmab Ag | Multiple Myeloma | Details |
| Catumaxomab | LP000 | Phase 3 Clinical | Trion Research, Neovii Biotech Gmbh | Ovarian Neoplasms; Stomach Neoplasms; Carcinoma; Neoplasms; Carcinoma, Ovarian Epithelial; Colonic Neoplasms; Urinary Bladder Neoplasms; Ascites; Breast Neoplasms | Details |
| Flotetuzumab | S-80880; MGD-006; RES-234 | Phase 2 Clinical | Macrogenics Inc | Leukemia, Myelogenous, Chronic; Hematologic Neoplasms; Leukemia; Leukemia, Hairy Cell; Mastocytosis, Systemic; Hodgkin Disease; Blastic Plasmacytoid Dendritic Cell Neoplasm; Precursor T-Cell Lymphoblastic Leukemia-Lymphoma; Neoplasms, Plasma Cell; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Leukemia, B-Cell; Leukemia, Myeloid, Acute; Leukemia, Biphenotypic, Acute | Details |
| Anti-CD3/humanized 3F8 bispecific antibody-activated T lymphocytes (University of Virginia) | Phase 2 Clinical | University Of Virginia | Neuroblastoma | Details | |
| Imvotamab | IGM-2323 | Phase 2 Clinical | Igm Biosciences Inc | Lymphoma, B-Cell, Marginal Zone; Myositis; Lupus Erythematosus, Cutaneous; Arthritis, Rheumatoid; Lymphoma, Large B-Cell, Diffuse; Lupus Erythematosus, Systemic; Lymphoma, Mantle-Cell; Lymphoma, Follicular; Arthritis; Lymphoma, Non-Hodgkin | Details |
| Foralumab | TZLS-401; NI-0401/α-CD3; NI-0401 | Phase 2 Clinical | Novimmune Sa, Bristol-Myers Squibb Company | Respiratory Tract Infections; Diabetes Mellitus, Type 2; Metabolic Dysfunction-Associated Steatotic Liver Disease; Coronavirus Disease 2019 (COVID-19); Multiple Sclerosis, Chronic Progressive; Multiple Sclerosis; Alzheimer Disease; Crohn Disease | Details |
| GNR-084 | GNR-084 | Phase 2 Clinical | Generium Pharmaceuticals, Iontas | Precursor Cell Lymphoblastic Leukemia-Lymphoma; Precursor B-Cell Lymphoblastic Leukemia-Lymphoma | Details |
| Anti-CD3 and anti-HER2 BiTE-expressing T cell (MedImmune) | Phase 2 Clinical | University Of Michigan, Huazhong University Of Science And Technology, University Of Virginia Cancer Center, Medimmune | Breast Neoplasms | Details | |
| Vibecotamab | XmAb-14045 | Phase 2 Clinical | Xencor Inc | Leukemia, Myeloid; Myelodysplastic Syndromes; Blastic Plasmacytoid Dendritic Cell Neoplasm; Precursor B-Cell Lymphoblastic Leukemia-Lymphoma; Leukemia, Myeloid, Acute | Details |
| α/β CD3+/CD19+ cell depleted stem cell therapy (Mitchell Cairo) | Phase 2 Clinical | New York Medical College | Leukemia; Anemia; Thalassemia; Hodgkin Disease; Anemia, Aplastic; Thrombocytopenia; Lymphoma, Non-Hodgkin; Kostmann Syndrome; Anemia, Sickle Cell | Details | |
| MK-6070 | HPN-328; MK-6070 | Phase 2 Clinical | Harpoon Therapeutics | Small Cell Lung Carcinoma | Details |
| Cibisatamab | CEA-TCB; RG-7802; RO-6958688; CEA-CD3 TCB | Phase 2 Clinical | F. Hoffmann-La Roche Ltd | Solid tumours; Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| CD3/CD19 neg allogeneic BMT (National Institute of Allergy and Infectious Diseases/University of Pittsburgh) | Phase 2 Clinical | National Institute Of Allergy And Infectious Diseases (Niaid), University Of Pittsburgh | Primary Immunodeficiency Diseases; Female Urogenital Diseases; Inflammation | Details | |
| HPN-536 | HPN-536 | Phase 2 Clinical | Harpoon Therapeutics | Neoplasms | Details |
| MBS-303 | MBS-303 | Phase 2 Clinical | Beijing Mabworks Biotech Co Ltd | Lymphoma, B-Cell; Lymphoma, Non-Hodgkin | Details |
| HBM-7022 | HBM-7022; AZD-5863 | Phase 2 Clinical | Harbour Biomed | Esophageal Neoplasms; Stomach Neoplasms; Pancreatic Neoplasms; Neoplasms; Digestive System Neoplasms; Esophageal adenocarcinoma; Carcinoma, Pancreatic Ductal; Gastrointestinal Neoplasms | Details |
| TAK-280 | MVC-280; TAK-280 | Phase 2 Clinical | Maverick Therapeutics Inc | Solid tumours; Neoplasms; Neoplasm Metastasis | Details |
| GB-261 | GB-261 | Phase 2 Clinical | Genor Biopharma Co Ltd | Lymphoma, B-Cell; Leukemia, Lymphoid; Lymphoma, Non-Hodgkin; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
| REGN-5459 | REGN-5459 | Phase 2 Clinical | Multiple Myeloma; Renal Insufficiency, Chronic | Details | |
| IBI-3003 | IBI3003; IBI-3003 | Phase 2 Clinical | Innovent Biologics(Suzhou) Co Ltd | Multiple Myeloma | Details |
| MBS-314 | MBS-314 | Phase 2 Clinical | Beijing Mabworks Biotech Co Ltd | Multiple Myeloma | Details |
| RO7515629 | RO7515629; RO-7515629; RG-6353 | Phase 2 Clinical | F. Hoffmann-La Roche Ag | Ovarian Neoplasms; Carcinoma, Renal Cell; Pancreatic Neoplasms; Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| BI-764532 | BI-764532; OBT620 | Phase 2 Clinical | C.H. Boehringer Sohn Ag & Co. Kg | Small Cell Lung Carcinoma; Neoplasms; Neuroendocrine Tumors; Carcinoma, Neuroendocrine; Glioma | Details |
| GEN3017 | GEN-3017 | Phase 2 Clinical | Genmab A/S | Hematologic Neoplasms; Hodgkin Disease; Lymphoma, Non-Hodgkin | Details |
| MP0533 | MP0533 | Phase 2 Clinical | Molecular Partners Ag | Leukemia, Myeloid, Acute | Details |
| CD30 biAb-AATC(The Medical College Of Wisconsin Nonprofit) | Phase 2 Clinical | The Medical College Of Wisconsin Nonprofit | Lymphoma, B-Cell; Leukemia; Hodgkin Disease; Lymphoma; Lymphoma, Large-Cell, Anaplastic; Lymphoma, T-Cell, Cutaneous | Details | |
| APVO-436 | APVO-436; APVO436 | Phase 2 Clinical | Aptevo | Myelodysplastic Syndromes; Leukemia, Myeloid, Acute | Details |
| Emfizatamab | GNC-038 | Phase 2 Clinical | SystImmune | Solid tumours; Lymphoma, Large B-Cell, Diffuse; Lymphoma, Extranodal NK-T-Cell; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Lymphoma, Non-Hodgkin; Lymphoma, T-Cell; Central Nervous System Lymphoma | Details |
| GEN-1047 | GEN-1047 | Phase 2 Clinical | Genmab A/S | Carcinoma, Non-Small-Cell Lung | Details |
| REGN-4336 | REGN-4336 | Phase 2 Clinical | Prostatic Neoplasms, Castration-Resistant | Details | |
| CM-350 | CM-350 | Phase 2 Clinical | Keymed Biosciences Co Ltd | Solid tumours | Details |
| 1-A-46 | BR-110; 1A46; 1-A-46; CMG1A46; CMG1A-46; BR110 | Phase 2 Clinical | Chengdu Chimagen Biosciences Co Ltd, BioRay Pharmaceutical Co Ltd | Lymphoma, B-Cell; Neoplasms; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Lymphoma, Non-Hodgkin | Details |
| LBL-034 | LBL-034 | Phase 2 Clinical | Nanjing Leads Biolabs Co Ltd | Neoplasms; Multiple Myeloma | Details |
| LBL-033 | LBL-033 | Phase 2 Clinical | Nanjing Leads Biolabs Co Ltd | Neoplasms | Details |
| CM-336(Connaught Biomedical Technology) | CM-336 | Phase 2 Clinical | Keymed Biosciences Co Ltd | Multiple Myeloma | Details |
| ICP-B02 | CM-355; ICP-B02 | Phase 2 Clinical | Beijing Tiannuo Jiancheng Pharmaceutical Technology Co Ltd, Keymed Biosciences Co Ltd | Hematologic Neoplasms; Hematoma; Lymphoma; Lymphoma, Non-Hodgkin | Details |
| SMET-12 | SMET-12 | Phase 2 Clinical | Zhejiang Shimai Pharmaceutical Co Ltd | Solid tumours; Carcinoma, Non-Small-Cell Lung | Details |
| QLS-31905 | QLS-31905 | Phase 2 Clinical | Qilu Pharmaceutical Co Ltd | Solid tumours; Neoplasms | Details |
| Nebratamig | GNC-035 | Phase 2 Clinical | Solid tumours; Hematologic Neoplasms; Breast Neoplasms; Metastatic breast cancer; Lymphoma, Non-Hodgkin; Leukemia, Lymphocytic, Chronic, B-Cell | Details | |
| TAK-186 | EGFR x CD3 COBRA; MVC-101; TAK-186 | Phase 2 Clinical | Takeda Pharmaceutical Co Ltd | Solid tumours; Squamous Cell Carcinoma of Head and Neck; Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| EMB-06 | EMB-06; EMB06 | Phase 2 Clinical | Shanghai Epimab Biotherapeutics, Inc | Multiple Myeloma | Details |
| AZD-0486 | TNB-486; AZD-0486; AZD0486 | Phase 2 Clinical | Teneobio Inc | Lymphoma, B-Cell; Lymphoma, Large B-Cell, Diffuse; Lymphoma, Follicular; Precursor B-Cell Lymphoblastic Leukemia-Lymphoma; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Leukemia, Myeloid, Acute; Lymphoma, Non-Hodgkin | Details |
| BNT-142 | BNT-142 | Phase 2 Clinical | Biontech Se | Solid tumours | Details |
| CN-201 | CN-201 | Phase 2 Clinical | Curon Biopharmaceutical Ltd | Lymphoma, B-Cell; Precursor B-Cell Lymphoblastic Leukemia-Lymphoma; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Leukemia, B-Cell; Lymphoma, Non-Hodgkin | Details |
| ZG006 | ZG006; ZG-006 | Phase 2 Clinical | Gensun Biopharma Inc, Suzhou Zelgen Biopharmaceuticals Co Ltd | Solid tumours; Neoplasms; Small Cell Lung Carcinoma; Carcinoma, Neuroendocrine | Details |
| EX-103 | EX-103; EX103 | Phase 2 Clinical | Guangzhou Excelmab Inc | Arthritis, Rheumatoid; Lymphoma, Non-Hodgkin; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
| Anti-CD3 monoclonal antibody (Jinan Tiankang Biological Products) | Phase 2 Clinical | Jinan Tiankang Biological Products Co Ltd | Anemia, Aplastic | Details | |
| Cevostamab | RG-6160; BFCR-4350A; RO-7187797 | Phase 2 Clinical | Genentech Inc | Multiple Myeloma | Details |
| Anti-CD3/anti-EGFR -activated T cells (Barbara Ann Karmanos Cancer Institute) | Phase 2 Clinical | Barbara Ann Karmanos Cancer Institute, University Of Virginia | Glioblastoma; Pancreatic Neoplasms | Details | |
| Ubamatamab | REGN-4018 | Phase 2 Clinical | Ovarian Neoplasms; Peritoneal Neoplasms; Endometrial Neoplasms; Fallopian Tube Neoplasms | Details | |
| Anti-CD3-anti-HER2-activated T cells | Phase 2 Clinical | Transtarget | Prostatic Neoplasms, Castration-Resistant; Breast Neoplasms; Prostatic Neoplasms | Details | |
| JCAR-014 | JCAR-014; JCAR021 | Phase 2 Clinical | Fred Hutchinson Cancer Research Center, Memorial Sloan Kettering Cancer Center, Seattle Children'S Research Institute, Juno Therapeutics Inc | Lymphoma, B-Cell; Leukemia; Leukemia, Lymphoid; Hodgkin Disease; Lymphoma, Large B-Cell, Diffuse; Candidiasis; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Lymphoma, Mantle-Cell; Primary mediastinal B cell lymphoma; Leukemia, B-Cell; Lymphoma, Non-Hodgkin; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
| A-319 | A-319 | Phase 1 Clinical | Evive Biotech Ltd | Lymphoma, B-Cell; Precursor B-Cell Lymphoblastic Leukemia-Lymphoma; Leukemia, B-Cell | Details |
| BCMA TriTAC | HPN-217 | Phase 1 Clinical | Harpoon Therapeutics, Abbvie Inc | Multiple Myeloma | Details |
| Membrane bound ligand T-SIGn virus | NG-348 | Phase 1 Clinical | Akamis Bio Ltd | Neoplasms | Details |
| NG-641 | EnAd-FAP-BiTE; EnAd-FAP-Tac; NG-641; NG-aFAP | Phase 1 Clinical | Akamis Bio Ltd, University Of Oxford | Squamous Cell Carcinoma of Head and Neck; Neoplasms, Glandular and Epithelial; Neoplasm Metastasis | Details |
| Vixtimotamab | T-564; AMV-564 | Phase 1 Clinical | Amphivena Therapeutics Inc | Solid tumours; Myelodysplastic Syndromes | Details |
| MGD-014 | MGD-014 | Phase 1 Clinical | Macrogenics Inc | HIV Infections | Details |
| JNJ-63898081 | JNJ-8081; JNJ-63898081 | Phase 1 Clinical | Johnson & Johnson Innovative Medicine | Neoplasms | Details |
| K-193 | K-193 | Phase 1 Clinical | Beijing Lvzhu Biological Technology Co Ltd | Lymphoma, B-Cell | Details |
| Anti-EGFR-bispecific antibody armed activated T-cell therapy (Memorial Sloan Kettering Cancer Center) | Phase 1 Clinical | Memorial Sloan Kettering Cancer Center | Pancreatic Neoplasms | Details | |
| Runimotamab | BTRC-4017A; RG-6194; RO-7227780 | Phase 1 Clinical | F. Hoffmann-La Roche Ltd | Solid tumours; Neoplasms | Details |
| Autologous T-cell therapy (anti-PSMA-CD3), Roger Williams Medical Center | Phase 1 Clinical | Roger Williams Medical Center | Prostatic Neoplasms | Details | |
| ONO-4685 | ONO-4685 | Phase 1 Clinical | Merus Nv | Psoriasis; Lymphoma, T-Cell; Plaque psoriasis | Details |
| WVT-078(Novartis Pharma) | WVT-078 | Phase 1 Clinical | Novartis Pharma Ag | Multiple Myeloma | Details |
| M-802(Wuhan Yzy Biopharma/CSPC Pharmaceutical) | M-802 | Phase 1 Clinical | Wuhan Yzy Biopharma Co Ltd, CSPC Pharmaceutical Group Ltd | Ovarian Neoplasms; Solid tumours; Stomach Neoplasms; Breast Neoplasms | Details |
| Emirodatamab | AMG-427 | Phase 1 Clinical | Amgen Inc | Leukemia, Myeloid, Acute | Details |
| JNJ-63709178 | JNJ-9178; CNTO-9958; JNJ-63709178 | Phase 1 Clinical | Johnson & Johnson Innovative Medicine, Genmab A/S | Leukemia, Myeloid, Acute | Details |
| SAR-442257 | SAR-442257 | Phase 1 Clinical | Sanofi | Neoplasms | Details |
| CC-1 | CC-1 | Phase 1 Clinical | University Of Tubingen, German Cancer Research Center (Dkfz) | Prostatic Neoplasms, Castration-Resistant; Prostatic Neoplasms; Carcinoma, Squamous Cell | Details |
| Acapatamab | AMG-160 | Phase 1 Clinical | Amgen Inc | Prostatic Neoplasms, Castration-Resistant; Carcinoma, Non-Small-Cell Lung | Details |
| Pavurutamab | AMG-701 | Phase 1 Clinical | Amgen Inc | Multiple Myeloma | Details |
| Xaluritamig | AMG-509 | Phase 1 Clinical | Xencor Inc, Amgen Inc | Prostatic Neoplasms, Castration-Resistant | Details |
| CC-3 | CC-3 | Phase 1 Clinical | Eberhard Karls University Of Tubingen, Germany | Colorectal Neoplasms; Gastrointestinal Neoplasms | Details |
| JS-203 | JS-203 | Phase 1 Clinical | Shanghai Junshi Biological Engineering Co Ltd | Lymphoma, B-Cell; Lymphoma, Non-Hodgkin | Details |
| BC-004 | BC-004 | Phase 1 Clinical | Shandong Buchang Pharmaceuticals Co Ltd | Solid tumours; Breast Neoplasms | Details |
| JY-016 | JY-016 | Phase 1 Clinical | Beijing Jingyitaixiang Technology Development Co Ltd, Beijing Eastern Biotech Co Ltd | Solid tumours; Colorectal Neoplasms; Lung Neoplasms | Details |
| EX-105 | EX-105; EX105 | Phase 1 Clinical | Guangzhou Excelmab Inc | Solid tumours | Details |
| EMB-07 | EMB-07 | Phase 1 Clinical | Shanghai Epimab Biotherapeutics, Inc | Solid tumours; Ovarian Neoplasms; Stomach Neoplasms; Neoplasms; Triple Negative Breast Neoplasms; Adenocarcinoma of Lung; Pancreatic Neoplasms; Urinary Bladder Neoplasms; Prostatic Neoplasms; Colorectal Neoplasms; Lymphoma; Uterine Neoplasms; Neoplasm Metastasis | Details |
| BA-3182 | BA-3182 | Phase 1 Clinical | Bioatla | Solid tumours; Adenocarcinoma | Details |
| BA-1202 | BA-1202 | Phase 1 Clinical | Solid tumours | Details | |
| CLN-978 | CLN-978 | Phase 1 Clinical | Adimab LLC | Precursor B-Cell Lymphoblastic Leukemia-Lymphoma; Lymphoma, Non-Hodgkin | Details |
| MGD-024 | MGD-024 | Phase 1 Clinical | Macrogenics Inc | Leukemia, Myelogenous, Chronic; Leukemia, Hairy Cell; Hodgkin Disease; Myelodysplastic Syndromes; Neoplasms; Blastic Plasmacytoid Dendritic Cell Neoplasm; Leukemia, B-Cell; Leukemia, Myeloid, Acute | Details |
| ARB-202 | ARB-202 | Phase 1 Clinical | Arbele Corp | Liver Neoplasms; Biliary Tract Neoplasms; Stomach Neoplasms; Pancreatic Neoplasms; Cholangiocarcinoma; Colorectal Neoplasms; Esophageal adenocarcinoma; Gastrointestinal Neoplasms | Details |
| CX-904 | CX-904 | Phase 1 Clinical | Amgen Inc, Cytomx Therapeutics Inc | Solid tumours; Neoplasms | Details |
| CLN-049 | CLN-049 | Phase 1 Clinical | University Of Tubingen, German Cancer Research Center (Dkfz) | Myelodysplastic Syndromes; Leukemia, Myeloid, Acute | Details |
| XmAb-819 | XmAb-819 | Phase 1 Clinical | Xencor Inc | Kidney Neoplasms; Carcinoma, Renal Cell | Details |
| JNJ-70218902 | JNJ-70218902 | Phase 1 Clinical | Johnson & Johnson Innovative Medicine | Neoplasms | Details |
| JNJ-67571244 | JNJ-67571244; JNJ-67371244; JNJ-1244 | Phase 1 Clinical | Johnson & Johnson | Myelodysplastic Syndromes; Leukemia, Myeloid, Acute | Details |
| SCTB-35 | SCTB35; SCTB-35 | Phase 1 Clinical | SinoCelltech Ltd | Lymphoma, B-Cell | Details |
| JNJ-87890387 | JNJ-87890387 | Phase 1 Clinical | Janssen Research & Development Llc | Solid tumours | Details |
| XmAb-541 | XmAb541; XmAb-541 | Phase 1 Clinical | Xencor Inc | Ovarian Neoplasms; Ovarian germ cell tumor; Germinoma; Endometrial Neoplasms; Neoplasms, Germ Cell and Embryonal | Details |
| [89Zr]Zr-BI-764532 | Phase 1 Clinical | C.H. Boehringer Sohn Ag & Co. Kg, Boehringer Ingelheim Gmbh | Small Cell Lung Carcinoma; Carcinoma, Neuroendocrine | Details | |
| TGI-6 | TGI-6 | Phase 1 Clinical | Hefei TG ImmunoPharma Co Ltd | Solid tumours; Colorectal Neoplasms | Details |
| SIM-0500 | SIM-0500; SIM0500 | Phase 1 Clinical | Hainan Xiansheng Re Ming Pharmaceutical Co Ltd | Bone Marrow Neoplasms; Multiple Myeloma | Details |
| Recombinant anti BCMA/CD3 bispecific antibody(Hualan Genetic Engineering) | Phase 1 Clinical | Hualan Genetic Engineering (Henan) Co Ltd | Multiple Myeloma | Details | |
| Oncolytic Virus R130(Yunying Medical) | R-130-OV; R130; R-130 | Phase 1 Clinical | Shanghai Yunying Medical Technology Co Ltd | Breast Neoplasms; Carcinoma, Non-Small-Cell Lung; Gastrointestinal Neoplasms; Melanoma; Laryngeal Neoplasms; Pharyngeal Neoplasms; Lung Neoplasms; Fallopian Tube Neoplasms; Colorectal Neoplasms; Genital Neoplasms, Female; Bone Neoplasms; Peritoneal Neoplasms; Sarcoma; Osteosarcoma; Head and Neck Neoplasms; Brain Neoplasms; Otorhinolaryngologic Neoplasms; Small Cell Lung Carcinoma; Pancreatic Neoplasms; Nose Neoplasms; Stomach Neoplasms; Esophageal Neoplasms; Carcinoma; Carcinoma, Bronchogenic; Liver Neoplasms; Ovarian Neoplasms; Kidney Neoplasms | Details |
| ASP2074 | ASP-2074; ASP2074 | Phase 1 Clinical | Astellas Pharma Global Development Inc | Solid tumours | Details |
| OBT620 | OBT620 | Phase 1 Clinical | Boehringer Ingelheim Gmbh, Oxford Biotherapeutics Ltd | Small Cell Lung Carcinoma | Details |
| CBA-1535 | CBA-1535 | Phase 1 Clinical | Chiome Bioscience Inc | Small Cell Lung Carcinoma; Triple Negative Breast Neoplasms; Mesothelioma; Carcinoma, Non-Small-Cell Lung | Details |
| JANX-008 | EGFR-TRACTr; JANX008; JANX-008 | Phase 1 Clinical | Janux Therapeutics Inc | Solid tumours; Squamous Cell Carcinoma of Head and Neck; Carcinoma, Renal Cell; Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| JANX-007 | JANX-007; PSMA-TRACTr | Phase 1 Clinical | Janux Therapeutics Inc | Prostatic Neoplasms, Castration-Resistant; Prostatic Neoplasms | Details |
| TQB-2934 | TQB2934; TQB-2934 | Phase 1 Clinical | Nanjing Shunxin Pharmaceuticals Co Ltd Of Chiatai Tianqing Pharmaceutical Group | Multiple Myeloma | Details |
| JNJ-80948543 | JNJ-80948543 | Phase 1 Clinical | Janssen Research & Development Llc | Lymphoma, B-Cell; Neoplasms; Lymphoma, Non-Hodgkin; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
| PIT-565 | PIT-565 | Phase 1 Clinical | Novartis Pharma Ag | Lymphoma, B-Cell; Precursor B-Cell Lymphoblastic Leukemia-Lymphoma; Lupus Erythematosus, Systemic; Leukemia, Myeloid, Acute | Details |
| CC-312 | CC-312 | Phase 1 Clinical | CytoCares (Shanghai) Inc | Hematologic Neoplasms; Lymphoma, B-Cell; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Lymphoma, Non-Hodgkin; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
| ASP-2138 | ASP-2138 | Phase 1 Clinical | Astellas Pharma Global Development Inc, Xencor Inc | Stomach Neoplasms; Esophageal Neoplasms; Pancreatic Neoplasms | Details |
| ISB-2001 | ISB-2001 | Phase 1 Clinical | Ichnos Sciences Sa | Multiple Myeloma | Details |
| GR-1901 | GR1901; GR-1901 | Phase 1 Clinical | Chongqing Zhixiang Jintai Biopharmaceutical Co Ltd, Genrix (Shanghai) Biopharmaceutical Co Ltd | Leukemia, Myeloid, Acute | Details |
| AMG-794 | AMG-794 | Phase 1 Clinical | Amgen Inc | Carcinoma, Ovarian Epithelial; Carcinoma, Non-Small-Cell Lung | Details |
| QLS-31904 | QLS31904; QLS-31904 | Phase 1 Clinical | Qilu Pharmaceutical Co Ltd | Solid tumours | Details |
| YK-012 | YK012 | Phase 1 Clinical | Lymphoma, B-Cell | Details | |
| RO-7428731 | RO-7428731; RG-6156 | Phase 1 Clinical | F. Hoffmann-La Roche Ltd | Glioblastoma | Details |
| GBR-1342 | GBR-1342; ISB-1342 | Phase 1 Clinical | Glenmark Pharmaceuticals Ltd | Multiple Myeloma | Details |
| Recombinant humanized anti-CD19/CD3 bispecific antibody(New Time Pharmaceutical) | LNF-1904 | Phase 1 Clinical | Shandong New Time Pharmaceutical Co Ltd | Lymphoma, B-Cell; Leukemia, B-Cell | Details |
| XmAb-968 | XmAb968 | Phase 1 Clinical | Xencor Inc | Hematologic Neoplasms; Leukemia, Promyelocytic, Acute | Details |
| GR-1803 | GR-1803 | Phase 1 Clinical | Chongqing Zhixiang Jintai Biopharmaceutical Co Ltd, Genrix (Shanghai) Biopharmaceutical Co Ltd | Multiple Myeloma | Details |
| XmAb-18968 | XmAb-18968 | Phase 1 Clinical | The Medical College Of Wisconsin Nonprofit | Leukemia, Myeloid, Acute | Details |
| SQZ-622(Novartis Pharma) | SQZ-622 | Phase 1 Clinical | Novartis Pharma Ag | Leukemia, Myeloid, Acute | Details |
| Forimtamig | RG-6234 | Phase 1 Clinical | F. Hoffmann-La Roche Ltd | Multiple Myeloma | Details |
| RG-6232 | RG-6232 | Phase 1 Clinical | F. Hoffmann-La Roche Ltd | Melanoma | Details |
| RG-6007 | RO-7283420; RG-6007 | Phase 1 Clinical | F. Hoffmann-La Roche Ltd | Leukemia, Myeloid, Acute | Details |
| SAR-443216 | SAR-443216 | Phase 1 Clinical | Sanofi | Solid tumours; Stomach Neoplasms; Neoplasms; Breast Neoplasms; Lung Neoplasms | Details |
| Recombinant oncolytic type II herpes simplex virus (Binhui Biopharmaceutical) | BS-006; oHSV2-BiTEs | Phase 1 Clinical | Wuhan Binhui Biotechnology Co Ltd | Solid tumours; Neoplasms; Melanoma; Uterine Cervical Neoplasms | Details |
| JNJ-78306358 | JNJ-78306358; JNJ-6358 | Phase 1 Clinical | Janssen Research & Development Llc | Solid tumours | Details |
| F-182112 | F-182112; F182112 | Phase 1 Clinical | Shandong New Time Pharmaceutical Co Ltd | Multiple Myeloma | Details |
| TNB-585 | TNB-585; AMG-340 | Phase 1 Clinical | Teneobio Inc | Prostatic Neoplasms, Castration-Resistant | Details |
| BC-3448 | BC3448; BC-3448 | Phase 1 Clinical | Solid tumours | Details | |
| IBI-389 | IBI-389 | Phase 1 Clinical | Innovent Biologics(Suzhou) Co Ltd | Solid tumours; Neoplasms | Details |
| hEGFRvIII-CD3 Bi-scFv | Phase 1 Clinical | Duke University | Glioblastoma; Glioma | Details | |
| JNJ-78278343 | Phase 1 Clinical | Janssen Research & Development Llc | Prostatic Neoplasms, Castration-Resistant; Prostatic Neoplasms | Details | |
| TQB-2825 | TQB-2825 | Phase 1 Clinical | Wuxi Biologics Co Ltd | Hematologic Neoplasms | Details |
| RGV-004 | RGV-004 | Phase 1 Clinical | Hangzhou Rongu Biotechnology Co Ltd | Lymphoma, B-Cell | Details |
| Recombinant humanized anti-BCMA/CD3 bispecific antibody(New Time Pharmaceutical) | Phase 1 Clinical | Shandong New Time Pharmaceutical Co Ltd | Multiple Myeloma | Details | |
| NVG-111 | NVG-111 | Phase 1 Clinical | NovalGen Ltd | Lymphoma, Large B-Cell, Diffuse; Lymphoma, Mantle-Cell; Lymphoma, Follicular; Melanoma; Carcinoma, Non-Small-Cell Lung; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
| BI-765049 | BI-765049; OBT-624 | Phase 1 Clinical | Oxford Biotherapeutics Ltd | Solid tumours | Details |
| GNC-039 | GNC-039 | Phase 1 Clinical | Solid tumours; Hematologic Neoplasms; Glioma; Neoplasm Metastasis | Details | |
| RO-7425781 | RO-7425781 | Phase 1 Clinical | F. Hoffmann-La Roche Ltd | Multiple Myeloma | Details |
| RO-7293583 | RO-7293583 | Phase 1 Clinical | F. Hoffmann-La Roche Ltd | Skin Melanoma; Uveal melanoma; Melanoma | Details |
| Rezetamig | JNJ-8780; JNJ75348780; JNJ-75348780 | Phase 1 Clinical | Teneobio Inc | Lymphoma, B-Cell; Lymphoma, Non-Hodgkin; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
| Y-150(Wuhan Yzy Biopharma/CSPC Pharmaceutical) | Y-150; Y150 | Phase 1 Clinical | Wuhan Yzy Biopharma Co Ltd, CSPC Pharmaceutical Group Ltd | Multiple Myeloma | Details |
| EX-101 | EX-101; EX101 | Phase 1 Clinical | Guangzhou Excelmab Inc | Solid tumours | Details |
| Plamotamab | XmAb-13676 | Phase 1 Clinical | Xencor Inc | Lymphoma, B-Cell; Lymphoma, Large B-Cell, Diffuse; Lymphoma, Non-Hodgkin; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
| A-337 | A-337 | Phase 1 Clinical | Evive Biotech Ltd | Solid tumours; Neoplasms | Details |
| CCW-702 | CCW-702 | Phase 1 Clinical | The Scripps Research Institute Inc, Abbvie Inc | Prostatic Neoplasms | Details |
| Tepoditamab | MCLA-117 | Phase 1 Clinical | Pharmaceutical Research Associates, Institute Gustave-Roussy, Merus Nv, Vu University Medical Center, Lgc | Leukemia, Myeloid, Acute | Details |
| ERY-974 | ERY-974 | Phase 1 Clinical | Chugai Pharmaceutical Co Ltd | Solid tumours; Carcinoma, Hepatocellular | Details |
| InHeAb-01 | InHeAb-01; bsAB | Clinical | University Hospital Tuebingen | Neoplasms | Details |
This web search service is supported by Google Inc.





