



























 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!
 Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!  Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!

This protein carries a mouse IgG2a Fc tag at the C-terminus
The protein has a calculated MW of 110.1 kDa. The protein migrates as 120-135 kDa under reducing (R) condition (SDS-PAGE) due to glycosylation.
>95% as determined by SDS-PAGE.
Lyophilized from 0.22 μm filtered solution in 50 mM Tris, 100 mM Glycine, pH7.5 with trehalose as protectant.
Contact us for customized product form or formulation.
Please see Certificate of Analysis for specific instructions.
For best performance, we strongly recommend you to follow the reconstitution protocol provided in the CoA.
For long term storage, the product should be stored at lyophilized state at -20°C or lower.
Please avoid repeated freeze-thaw cycles.
This product is stable after storage at:
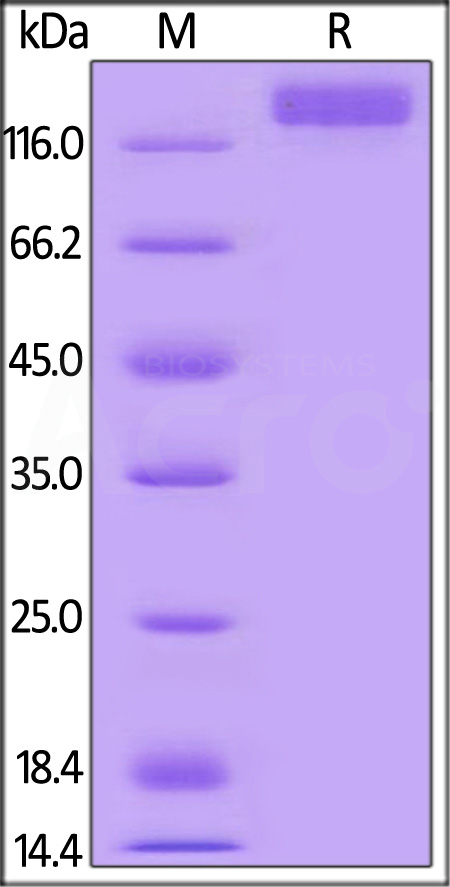
Mouse VEGF R2 Protein, Mouse IgG2a Fc Tag on SDS-PAGE under reducing (R) condition. The gel was stained with Coomassie Blue. The purity of the protein is greater than 95%.

Immobilized Human VEGF165, premium grade (Cat. No. VE5-H4210) at 2 μg/mL (100 μL/well) can bind Mouse VEGF R2 Protein, Mouse IgG2a Fc Tag (Cat. No. VE2-M5258) with a linear range of 1-31 ng/mL (QC tested).
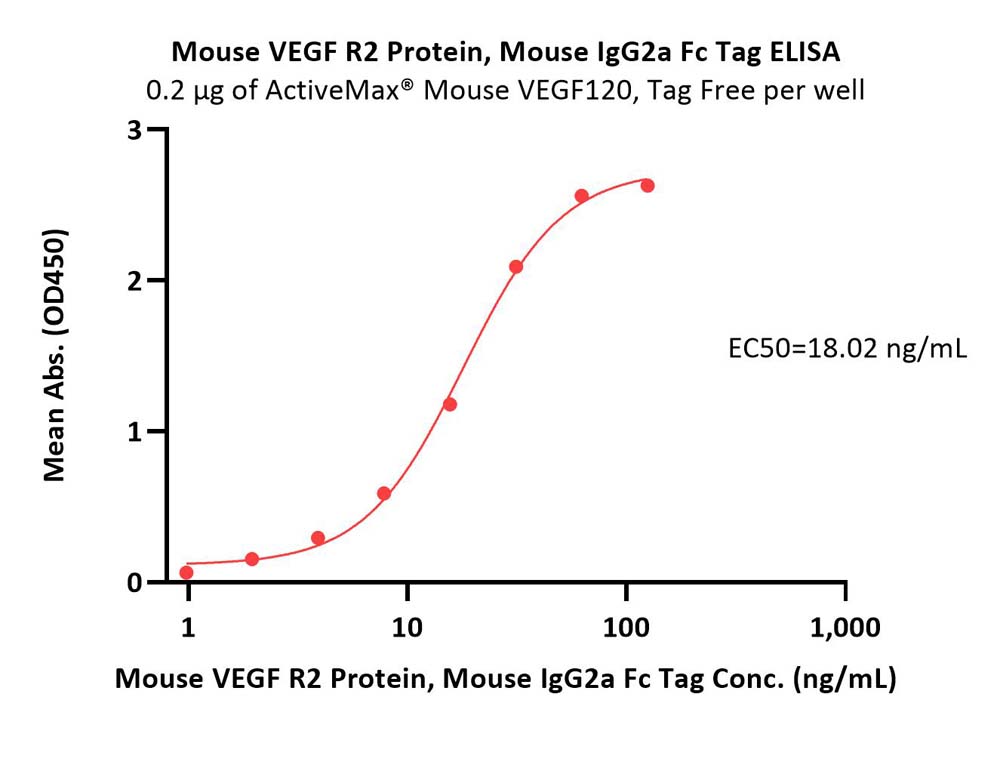
Immobilized ActiveMax® Mouse VEGF120, Tag Free (Cat. No. VE0-M4211) at 2 μg/mL (100 μL/well) can bind Mouse VEGF R2 Protein, Mouse IgG2a Fc Tag (Cat. No. VE2-M5258) with a linear range of 1-31 ng/mL (Routinely tested).
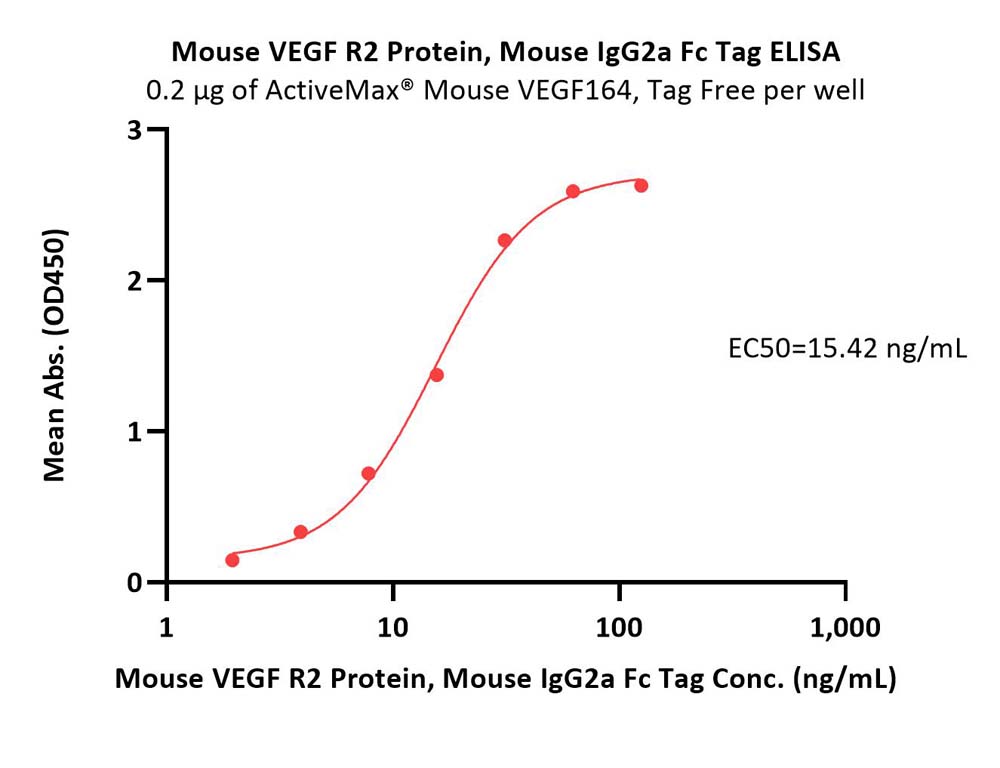
Immobilized ActiveMax® Mouse VEGF164, Tag Free (Cat. No. VE4-M4216) at 2 μg/mL (100 μL/well) can bind Mouse VEGF R2 Protein, Mouse IgG2a Fc Tag (Cat. No. VE2-M5258) with a linear range of 2-31 ng/mL (Routinely tested).

Loaded Biotinylated Mouse VEGF164, His,Avitag (Cat. No. VE4-M82Q3) on SA Biosensor, can bind Mouse VEGF R2 Protein, Mouse IgG2a Fc Tag (Cat. No. VE2-M5258) with an affinity constant of 1.33 nM as determined in BLI assay (ForteBio Octet Red96e) (Routinely tested).

ACROBiosystems는 생체 활성이 높은 균질한 CD3 δ/CD3 ε 및 CD3 γ/CD3 ε 단백질을 개발하여 이중특이 항체를 치료제로의 임상 개발을 가속시킵니다.

We offer a wide range of cell and gene therapy solutions starting from discovery to the clinic. Explore our wide range of proteins, antibodies, kits, and other assays to accelerate the development of your cell and gene therapy.

오가노이드 툴 박스는 약물 개발 프로젝트의 진행을 가속할 수 있는 레디메이드 오가노이드와 오가노이드 분화 툴 키트 및 다양한 서비스를 포함하는 오가노이드 솔루션 모음입니다.

고품질 GMP 등급 사이토카인 IL-15,IL-7, IL-21 등 제품은 신속한 임상/신약승인신청을 위해 개발된 것입니다.

50+ car-t의 인기 타겟을 커버하고, car 발현 검사를 위해 특별히 설계된 항원 단백질은 스트림 검증을 통해 고특이성 car 발현을 검출할 수 있으며, 고 배치간 일관성을 가지고 있다.pe/fi표기 단백질로 한 단계 염색하면 고특이성, 무 백그라운드로 car 발현 검사를 검사할 수 있습니다.

cd20, claudin18.2, cd133, gprc5d, ccr5, ccr8을 포함한 안정적이고 활성이 높은 전장 다중 막관통 단백질은 면역, elisa, spr, bli, 세포 실험, car 양성률 검사 등에 광범위하게 사용된다. vlp, 스케일제거제, nanodisc의 다양한 기술 플랫폼은 다중 막관통 단백질을 목표로 하는 약물 연구에 도움을 준다.

GMP 등급 사이토카인. 고품질 세포 활성화 및 확장 시약. 유전자 편집 시약, 효소 CAR 검사 시약 등 제품. 세포 및 유전자 요법 고객님에게 포괄적인 솔루션을 제공하고 초기 약물 발견에서 임상 연구에 이르기까지 모든 단계에서 귀하와 동반하겠습니다.

현재 이미 알려진 거의 대부분의 면역 체크 포인트 분자를 포함하여 다양한 레벨과 물종을 제공하여 고객이 선택할수 있도록 하고있다. 천연중합체 형식은 mals를 통해 검증되었고 생물활성은 elisa/spr/bli/facs 등을 통해 검증되었으며 또한 biotin/fitc 표지는 높은 처리량을 가진 항체를 선별하는데 편리하다.

ACROBiosystems는 ADC약물의 연구개발을 돕기 위해 노력하고 있으며, 다양한 인기 타겟에 서로 다른 종, 다른 라벨의 제품을 개발했으며, 높은 순도, 높은 친화력 등 특징을 가지고 있으며, 면역, 항체 선택, spr, 세포 활성 검사 및 기타 실험에 적합하고, 해당 protocol을 무료로 제공합니다.

Fc 수용체 단백질은 모든 분자를 포함하고 있을 뿐만 아니라, 일반적인 돌연변이와 바이오틴 표기 종류을 포함하고 있어 고객들이 단일 클론 항체 개발을 가속화할 수 있다.

포괄적으로 풍부한 사이토카인 표적 단백질은 HEK293 진핵생물 시스템에 의해 발현되며 천연 구조에 더 가깝다. 고순도는 SDS-PAGE/HPLC/MALS에 의해 검증된다. 높은 생물학적 활성이 ELISA/SPR/BLI에 의해 검증된다. 배치간 일관성이 우수하여 항체 면역, 스크리닝 및 품질 관리 과정에 적합하다.

Aneuro는 ACROBiosystems가 뇌과학 연구에 초점을 맞춘 제품 라인으로서 치료 및 진단 연구 단백질, PFFs와 재조합 신경 인자 등 뇌과학 연구 분야에 중요한 단백질 제품을 제공하여 뇌과학 연구에 도움이 된다.
| English Name | Research Code | Research Phase | Company | First Brand Name | First Approved Country | First Indication | First Approved Company | First Approved Date | Indications | Clinical Trials |
|---|---|---|---|---|---|---|---|---|---|---|
| Anlotinib Dihydrochloride | AL-3818 | Approved | Advenchen Laboratories Llc, Jiangsu Chia Tai-Tianqing Pharmaceutical Co Ltd | 福可维 | Mainland China | Carcinoma, Non-Small-Cell Lung | Jiangsu Chia Tai-Tianqing Pharmaceutical Co Ltd | 2018-05-08 | Sarcoma, Alveolar Soft Part; Bile Duct Diseases; Colorectal Neoplasms; Gastrointestinal Stromal Tumors; Peritoneal Neoplasms; Hepatic Insufficiency; Bone Neoplasms; Urologic Neoplasms; Fallopian Tube Neoplasms; Thyroid Neoplasms; Endometrial Neoplasms; Medullary thyroid cancer (MTC); Gallbladder Neoplasms; Glioma; Lung Neoplasms; Esophageal Squamous Cell Carcinoma; Carcinoma, Non-Small-Cell Lung; Uterine Cervical Neoplasms; Osteoma; Melanoma; Carcinoma, Hepatocellular; Carcinoma, Ovarian Epithelial; Leiomyosarcoma; Solid tumours; Drug-Related Side Effects and Adverse Reactions; Biliary Tract Neoplasms; Head and Neck Neoplasms; Esophageal Neoplasms; Stomach Neoplasms; Thoracic Neoplasms; Carcinoma, Renal Cell; Ovarian Neoplasms; Small Cell Lung Carcinoma; Neoplasms; Lymphoma, Large B-Cell, Diffuse; Sarcoma, Synovial; Neuroendocrine Tumors; Lung Diseases, Interstitial; Liver Diseases; Sarcoma; Nasopharyngeal Carcinoma | Details |
| Lenvatinib Mesylate | MK-7902; ER-203492-00; E-7080 | Approved | Eisai Co Ltd | Kisplyx, Lenvima, Lenvima/Kisplyx, 乐卫玛 | United States | Thyroid Neoplasms | Eisai Inc | 2015-02-13 | Carcinoma, Adenoid Cystic; Paraganglioma; Melanoma; Thyroid Cancer, Papillary; Carcinoma, Hepatocellular; Neoplasm Metastasis; Carcinoma, Non-Small-Cell Lung; Glioma; Lymphoma; Endometrial Neoplasms; Thyroid Neoplasms; Hepatic Insufficiency; Breast Neoplasms; Cholangiocarcinoma; Osteosarcoma; Solid tumours; Neuroendocrine Tumors; Adenocarcinoma, Follicular; Liver Diseases; Thyroid Carcinoma, Anaplastic; Adenocarcinoma of Lung; Kidney Diseases; Neoplasms; Pheochromocytoma; Esophageal Neoplasms; Renal Insufficiency; Carcinoma, Renal Cell; Liver Neoplasms; Ovarian Neoplasms; Biliary Tract Neoplasms | Details |
| Axitinib | PF-01367866; AG-13736; AG-013736 | Approved | Pfizer Inc | 英立达, Inlyta | United States | Carcinoma, Renal Cell | Pf Prism Cv | 2012-01-27 | Lung Neoplasms; Carcinoma, Adenoid Cystic; Prostatic Neoplasms, Castration-Resistant; Breast Neoplasms; Sarcoma; Prostatic Neoplasms; Cholangiocarcinoma; Hepatic Insufficiency; Colorectal Neoplasms; Thyroid Neoplasms; Neuroendocrine Tumors; Leukemia, Myeloid, Acute; Carcinoma, Pancreatic Ductal; Lymphoma; Carcinoma, Hepatocellular; Adenocarcinoma; Carcinoma, Non-Small-Cell Lung; Melanoma; Paraganglioma; Myelodysplastic Syndromes; Ovarian Neoplasms; Solid tumours; Carcinoma, Renal Cell; Carcinoma; Pheochromocytoma; Squamous Cell Carcinoma of Head and Neck; Stomach Neoplasms; Carcinoid Tumor; Kidney Neoplasms; Neoplasms; Nasopharyngeal Neoplasms; Adrenal Cortex Neoplasms; Skin Neoplasms; Pancreatic Neoplasms; Glioblastoma; Mesothelioma; Carcinoma, Ductal | Details |
| Regorafenib | DAST; BAY-73-4506 | Approved | Bayer AG | Stivarga, Resihance | United States | Colorectal Neoplasms | Bayer Healthcare Pharmaceuticals Inc | 2012-09-27 | Fallopian Tube Neoplasms; Osteosarcoma; Sarcoma, Ewing; Colorectal Neoplasms; Gastrointestinal Stromal Tumors; Peritoneal Neoplasms; Bone Neoplasms; Bile Duct Neoplasms; Thymoma; Thyroid Neoplasms; Leukemia, Myeloid, Acute; Gastrinoma; Lung Neoplasms; Esophageal adenocarcinoma; Carcinoma, Hepatocellular; Carcinoma, Non-Small-Cell Lung; Melanoma; Gastrointestinal Neoplasms; Somatostatinoma; Adenocarcinoma; Neoplasm Metastasis; Meningioma; Neoplasms; Solid tumours; Ovarian Neoplasms; Rectal Neoplasms; Carcinoma, Renal Cell; Hemangiosarcoma; Carcinoid Tumor; Insulinoma; Carcinoma, Islet Cell; Stomach Neoplasms; Esophageal Neoplasms; Liver Neoplasms; Carcinoma, Transitional Cell; Colonic Neoplasms; Pancreatic Neoplasms; Glioblastoma; Carcinoma, Ovarian Epithelial; Adenoma; Glucagonoma; Carcinoma, Adenoid Cystic; Sarcoma | Details |
| Nintedanib Esylate | BIBF-1120 | Approved | C.H. Boehringer Sohn Ag & Co. Kg | Ofev, Vargatef | United States | Idiopathic Pulmonary Fibrosis | Boehringer Ingelheim Gmbh | 2014-10-15 | Lung Neoplasms; Breast Neoplasms; Prostatic Neoplasms; Adenocarcinoma, Clear Cell; systemic sclerosis-associated interstitial lung disease; Colorectal Neoplasms; Peritoneal Neoplasms; Gliosarcoma; Hepatic Insufficiency; Astrocytoma; Genital Neoplasms, Female; Silicosis; Sarcoma; Carcinoma, Squamous Cell; Fallopian Tube Neoplasms; Appendiceal Neoplasms; Leukemia, Myeloid, Acute; Uterine Neoplasms; Endometrial Neoplasms; Carcinoma, Non-Small-Cell Lung; Carcinoma, Hepatocellular; Adenocarcinoma; Carcinoma, Endometrioid; Scleroderma, Systemic; Telangiectasia, Hereditary Hemorrhagic; Solid tumours; Rejection of lung transplantation; Carcinoma, Renal Cell; Radiation Pneumonitis; Esophageal Neoplasms; Carcinoid Tumor; Endometrial Stromal Tumors; Idiopathic Pulmonary Fibrosis; Neoplasms; Ovarian Neoplasms; Glioblastoma; Colonic Neoplasms; Small Cell Lung Carcinoma; Pulmonary Fibrosis; Lung Diseases, Interstitial; Oligodendroglioma; Multiple Myeloma; Mesothelioma; Asbestosis; Neuroendocrine Tumors | Details |
| Vorolanib | X-82; EYP-1901; CM-082 | Approved | Tyrogenex Inc | 伏美纳 | Mainland China | Carcinoma, Renal Cell | Betta Pharmaceuticals Co Ltd | 2023-06-07 | Diabetic macular oedema; Carcinoma, Non-Small-Cell Lung; Melanoma; Macular Degeneration; Carcinoma, Hepatocellular; Diabetic Retinopathy; Choroidal Neovascularization; Adenocarcinoma; Retinal Degeneration; Leukemia, Myeloid, Acute; Eye Diseases; Solid tumours; Wet Macular Degeneration; Retinal Diseases; Small Cell Lung Carcinoma; Neoplasms; Pancreatic Neoplasms; Stomach Neoplasms; Esophageal Neoplasms; Carcinoma, Renal Cell; Kidney Neoplasms | Details |
| Tivozanib | Kil-8951; AV-951; KRN-951; ASP-4130; KHK-4951; KHK4951; UNII-172030934T | Approved | Kyowa Hakko Kirin Co Ltd | Fotivda, FOTIVDA | EU | Carcinoma, Renal Cell | Recordati Netherlands BV | 2017-08-24 | Colorectal Neoplasms; Carcinoma, Hepatocellular; Carcinoma, Non-Small-Cell Lung; Gastrointestinal Neoplasms; Macular Degeneration; Fallopian Tube Neoplasms; Metastatic breast cancer; Peritoneal Neoplasms; Hepatic Insufficiency; Bile Duct Neoplasms; Biliary Tract Neoplasms; Sarcoma; Breast Neoplasms; Cholangiocarcinoma; Carcinoma, Ovarian Epithelial; Triple Negative Breast Neoplasms; Carcinoma, Renal Cell; Ovarian Neoplasms; Liver Neoplasms; Solid tumours | Details |
| Apatinib Mesylate | YN-968D1 | Approved | Advenchen Laboratories Llc | 艾坦, Aitan | Mainland China | Stomach Neoplasms | Jiangsu shengdi Medicine Co Ltd | 2014-10-17 | Nasopharyngeal Neoplasms; Adenocarcinoma; Carcinoma, Hepatocellular; Carcinoma, Non-Small-Cell Lung; Lung Neoplasms; Fallopian Tube Neoplasms; Colorectal Neoplasms; Peritoneal Neoplasms; Nasopharyngeal Carcinoma; Breast Neoplasms; Carcinoma, Adenoid Cystic; Head and Neck Neoplasms; Triple Negative Breast Neoplasms; Carcinoma, Ovarian Epithelial; Neoplasms; Esthesioneuroblastoma, Olfactory; Esophageal Neoplasms; Stomach Neoplasms; Solid tumours; Ovarian Neoplasms; Liver Neoplasms; Biliary Tract Neoplasms | Details |
| Ripretinib | DCC-2618 | Approved | Deciphera | Qinlock, 擎乐 | United States | Gastrointestinal Stromal Tumors | Deciphera Pharmaceuticals Llc | 2020-05-15 | Neoplasms; Mastocytosis, Systemic; Gastrointestinal Stromal Tumors | Details |
| Ramucirumab | IMC-1121; IMC-1C11; LY-3009806; IMC-1121-B; BLA-125477 | Approved | Dyax Pharma | Cyramza | United States | Gastrointestinal Stromal Tumors | Eli Lilly And Company | 2014-04-21 | Breast Neoplasms; Melanoma; Carcinoma, Hepatocellular; Adenocarcinoma; Carcinoma, Non-Small-Cell Lung; Fallopian Tube Neoplasms; Ureteral Neoplasms; Urologic Neoplasms; Peritoneal Neoplasms; Gastrointestinal Stromal Tumors; Colorectal Neoplasms; Prostatic Neoplasms; Urethral Neoplasms; Liver Neoplasms; Pancreatic Neoplasms; Colonic Neoplasms; Carcinoma, Transitional Cell; Neoplasms; Carcinoma, Renal Cell; Rectal Neoplasms; Esophageal Neoplasms; Carcinoid Tumor; Stomach Neoplasms; Biliary Tract Neoplasms; Ovarian Neoplasms; Solid tumours | Details |
| Sunitinib Malate | PNU-290940AD; PHA-290940AD; SCAI-003; GB-102; PNU-290940; SU-011248-L-malate salt; PHA-290940; SU-010398; SU-11248 | Approved | Pfizer Inc | 索坦, Sutent | United States | Carcinoma, Renal Cell; Gastrointestinal Stromal Tumors | Cppi Cv | 2006-01-26 | Solid tumours; Fibromatosis, Aggressive; Ovarian Neoplasms; Kidney Neoplasms; Leukemia, Myelogenous, Chronic; Head and Neck Neoplasms; Leiomyosarcoma; Lymphoma, B-Cell; Lymphoma, B-Cell, Marginal Zone; HIV Infections; Leukemia, Myeloid, Accelerated Phase; Leukemia; Fibrosarcoma; Teratoma; Liver Neoplasms; Ependymoma; Lymphoma, T-Cell, Peripheral; Intestinal Neoplasms; Histiocytoma, Malignant Fibrous; Carcinoma, Renal Cell; Hemangioblastoma; Carcinoma, Islet Cell; Carcinoma; Pheochromocytoma; Stomach Neoplasms; Pelvic Neoplasms; Esophageal Neoplasms; Leukemia, Hairy Cell; Polycythemia Vera; Abdominal Neoplasms; Squamous Cell Carcinoma of Head and Neck; Thoracic Neoplasms; Pancreatic neuroendocrine tumors (pNET); Carcinoma, Ovarian Epithelial; Glioblastoma; Neurofibromatoses; Small Cell Lung Carcinoma; Adenoma, Islet Cell; Carcinoma, Transitional Cell; Pancreatic Neoplasms; Lymphoma, Large B-Cell, Diffuse; Neoplasms; Myelodysplastic Syndromes; Hodgkin Disease; Leukemia, Myelomonocytic, Chronic; Carcinoma, Papil | Details |
| Pazopanib Hydrochloride | GSK-786034; GW-786034B; SB-786034; GW-786034 | Approved | Glaxosmithkline Plc, Novartis Pharma Ag | 维全特, Armala, Votrient, Patorma | United States | Carcinoma, Renal Cell; Sarcoma | Novartis Pharma Ag | 2009-10-19 | Uterine Cervical Diseases; Fallopian Tube Neoplasms; Lung Neoplasms; Uterine Neoplasms; Choriocarcinoma; Lymphoma; Colorectal Neoplasms; Gastrointestinal Stromal Tumors; Carcinoma, Mucoepidermoid; Gliosarcoma; Genital Neoplasms, Female; Leukemia, Myeloid, Acute; Peritoneal Neoplasms; Brain Neoplasms; Urethral Neoplasms; Medullary thyroid cancer (MTC); Chondrosarcoma, Extraskeletal Myxoid; Prostatic Neoplasms; Osteosarcoma; Neuroblastoma; Sarcoma; Breast Neoplasms; Carcinoma, Hepatocellular; Uterine Cervical Neoplasms; Breast Neoplasms, Male; Neoplasm Metastasis; von Hippel-Lindau Disease; Thyroid Cancer, Papillary; Gastrointestinal Neoplasms; Paraganglioma; Endodermal Sinus Tumor; Neoplasms, Germ Cell and Embryonal; Melanoma; Gastrinoma; Macular Degeneration; Carcinoma, Non-Small-Cell Lung; Thyroid Neoplasms; Carcinoma, Squamous Cell; Carcinoma, Small Cell; Carcinoma, Embryonal; Germinoma; Glioma; Carcinoma, Neuroendocrine; Epistaxis; Carcinoma, Ovarian Epithelial; Neoplasms; Squamous Cell Carcinoma of Head a | Details |
| Cabozantinib S-malate | XL-184; BMS-907351 | Approved | Exelixis Inc | Cometriq, Cabometyx | United States | Carcinoma, Neuroendocrine; Thyroid Neoplasms | Exelixis Inc | 2012-11-29 | Urethral Neoplasms; Leukemia, Myeloid, Acute; Thyroid Neoplasms; Gliosarcoma; Colorectal Neoplasms; Astrocytoma; Hepatic Insufficiency; Peritoneal Neoplasms; Bile Duct Neoplasms; Sarcoma, Clear Cell; Uterine Neoplasms; Adenocarcinoma, Clear Cell; Sarcoma, Ewing; Carcinoma, Adenosquamous; Breast Neoplasms; Neurofibroma, Plexiform; Osteosarcoma; Prostatic Neoplasms; Sarcoma; Medullary thyroid cancer (MTC); Carcinoma, Hepatocellular; Melanoma; Paraganglioma; Meningioma; Neoplasms, Germ Cell and Embryonal; Neoplasm Metastasis; Carcinoma, Non-Small-Cell Lung; Adenocarcinoma; Carcinoma, Endometrioid; Thyroid Cancer, Papillary; Brain Neoplasms; Carcinoma, Neuroendocrine; Sarcoma, Alveolar Soft Part; Lymphoma; Fallopian Tube Neoplasms; Brain metastases; Glioma; Endometrial Neoplasms; Carcinoma, Squamous Cell; Carcinoid Tumor; Glioblastoma; Skin Neoplasms; Neoplasms; Carcinoma, Papillary; Hepatoblastoma; Pheochromocytoma; Pain; Rejection of liver transplantation; Carcinoma, Renal Cell; Pancreatic neuroendocrine tumors | Details |
| Fruquintinib | HMPL-013; TAK-113 | Approved | Hutchison Medipharma Ltd | Elunate, FRUZAQLA, Aiyoute, 爱优特 | Mainland China | Colorectal Neoplasms | Hutchison Medipharma Ltd | 2018-09-04 | Small Cell Lung Carcinoma; Carcinoma, Non-Small-Cell Lung; Endometrial Neoplasms; Lung Neoplasms; Hepatic Insufficiency; Colorectal Neoplasms; Breast Neoplasms; Sarcoma; Kidney Diseases; Neoplasms; Solid tumours; Triple Negative Breast Neoplasms; Pancreatic Neoplasms; Colonic Neoplasms; Rectal Neoplasms; Esophageal Neoplasms; Stomach Neoplasms; Carcinoma, Renal Cell; Biliary Tract Neoplasms | Details |
| Sorafenib Tosylate | NSC-724772; BAY-43-0006; BAY-43-9006; BAY-54-9085 | Approved | Onyx Pharmaceuticals Inc | Nexavar, 多吉美 | United States | Carcinoma, Renal Cell | Bayer Healthcare Pharmaceuticals Inc | 2005-12-01 | Liver Neoplasms; Kidney Neoplasms; Recurrence; Ovarian Neoplasms; Leukemia, Myelogenous, Chronic; Lymphoma, T-Cell, Peripheral; Leukemia, Erythroblastic, Acute; Rhabdomyosarcoma; Lymphoma, B-Cell, Marginal Zone; Fibromatosis, Aggressive; Head and Neck Neoplasms; Solid tumours; Leiomyosarcoma; Leukemia, Myeloid; Carcinoma, Renal Cell; Carcinoma; Vipoma; Esophageal Neoplasms; Hemangiosarcoma; Squamous Cell Carcinoma of Head and Neck; Rectal Neoplasms; Carcinoid Tumor; Histiocytoma, Malignant Fibrous; Carcinoma, Islet Cell; Insulinoma; Stomach Neoplasms; Neoplasms; Kidney Diseases; Small Cell Lung Carcinoma; Carcinoma, Transitional Cell; Carcinoma, Verrucous; Thyroid Carcinoma, Anaplastic; Myelodysplastic Syndromes; Glioblastoma; Pancreatic Neoplasms; Leukemia, Myelomonocytic, Chronic; Carcinoma, Ovarian Epithelial; Leukemia, Myelomonocytic, Acute; Wilms Tumor; Lymphomatoid Granulomatosis; Colonic Neoplasms; Hypertension, Portal; Lymphoma, Large B-Cell, Diffuse; Lymphoma, Large-Cell, Immunoblastic; Multiple Endo | Details |
| Vandetanib | AZD-6474; CH-331; ZD-6474 | Approved | Astrazeneca Plc | Zactima, Caprelsa | United States | Carcinoma, Neuroendocrine; Thyroid Neoplasms | Genzyme Corp | 2011-04-06 | Lung Neoplasms; Neuroblastoma; Medullary thyroid cancer (MTC); Prostatic Neoplasms; Urethral Neoplasms; Peritoneal Neoplasms; Gliosarcoma; Colorectal Neoplasms; Ureteral Neoplasms; Gastrointestinal Stromal Tumors; Diffuse Intrinsic Pontine Glioma; Lymphoproliferative Disorders; Astrocytoma; Thyroid Neoplasms; Pleural Effusion; Carcinoma, Squamous Cell; Lymphoma; Fallopian Tube Neoplasms; Multiple Endocrine Neoplasia Type 2b; Glioma; Carcinoma, Neuroendocrine; Carcinoma, Non-Small-Cell Lung; Paraganglioma; Precancerous Conditions; Neoplasm Metastasis; Adenocarcinoma; von Hippel-Lindau Disease; Carcinoma, Hepatocellular; Anus Neoplasms; Biliary Tract Neoplasms; Solid tumours; Kidney Neoplasms; Intestinal Neoplasms; Ovarian Neoplasms; Pheochromocytoma; Stomach Neoplasms; Esophageal Neoplasms; Carcinoma, Renal Cell; Carcinoma; Squamous Cell Carcinoma of Head and Neck; Rectal Neoplasms; Abdominal Neoplasms; Head and Neck Neoplasms; Small Cell Lung Carcinoma; Carcinoma, Transitional Cell; Pancreatic Neoplasms; Neop | Details |
| Midostaurin | PKC-412; PKC-412A; CGP-41231; CGP-41251 | Approved | Novartis Pharma Ag | Rydapt | United States | Mastocytosis, Systemic; Leukemia, Mast-Cell; Hematologic Neoplasms; Leukemia, Myeloid, Acute | Novartis Pharmaceuticals Corp | 2017-04-28 | Leukemia; Hematologic Neoplasms; Mastocytosis, Systemic; Myelodysplastic Syndromes; Leukemia, Mast-Cell; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Hepatic Insufficiency; Leukemia, Myeloid, Acute; Mastocytosis | Details |
| Anlotinib Dihydrochloride | AL-3818 | Approved | Advenchen Laboratories Llc, Jiangsu Chia Tai-Tianqing Pharmaceutical Co Ltd | 福可维 | Mainland China | Carcinoma, Non-Small-Cell Lung | Jiangsu Chia Tai-Tianqing Pharmaceutical Co Ltd | 2018-05-08 | Sarcoma, Alveolar Soft Part; Bile Duct Diseases; Colorectal Neoplasms; Gastrointestinal Stromal Tumors; Peritoneal Neoplasms; Hepatic Insufficiency; Bone Neoplasms; Urologic Neoplasms; Fallopian Tube Neoplasms; Thyroid Neoplasms; Endometrial Neoplasms; Medullary thyroid cancer (MTC); Gallbladder Neoplasms; Glioma; Lung Neoplasms; Esophageal Squamous Cell Carcinoma; Carcinoma, Non-Small-Cell Lung; Uterine Cervical Neoplasms; Osteoma; Melanoma; Carcinoma, Hepatocellular; Carcinoma, Ovarian Epithelial; Leiomyosarcoma; Solid tumours; Drug-Related Side Effects and Adverse Reactions; Biliary Tract Neoplasms; Head and Neck Neoplasms; Esophageal Neoplasms; Stomach Neoplasms; Thoracic Neoplasms; Carcinoma, Renal Cell; Ovarian Neoplasms; Small Cell Lung Carcinoma; Neoplasms; Lymphoma, Large B-Cell, Diffuse; Sarcoma, Synovial; Neuroendocrine Tumors; Lung Diseases, Interstitial; Liver Diseases; Sarcoma; Nasopharyngeal Carcinoma | Details |
| Lenvatinib Mesylate | MK-7902; ER-203492-00; E-7080 | Approved | Eisai Co Ltd | Kisplyx, Lenvima, Lenvima/Kisplyx, 乐卫玛 | United States | Thyroid Neoplasms | Eisai Inc | 2015-02-13 | Carcinoma, Adenoid Cystic; Paraganglioma; Melanoma; Thyroid Cancer, Papillary; Carcinoma, Hepatocellular; Neoplasm Metastasis; Carcinoma, Non-Small-Cell Lung; Glioma; Lymphoma; Endometrial Neoplasms; Thyroid Neoplasms; Hepatic Insufficiency; Breast Neoplasms; Cholangiocarcinoma; Osteosarcoma; Solid tumours; Neuroendocrine Tumors; Adenocarcinoma, Follicular; Liver Diseases; Thyroid Carcinoma, Anaplastic; Adenocarcinoma of Lung; Kidney Diseases; Neoplasms; Pheochromocytoma; Esophageal Neoplasms; Renal Insufficiency; Carcinoma, Renal Cell; Liver Neoplasms; Ovarian Neoplasms; Biliary Tract Neoplasms | Details |
| Axitinib | PF-01367866; AG-13736; AG-013736 | Approved | Pfizer Inc | 英立达, Inlyta | United States | Carcinoma, Renal Cell | Pf Prism Cv | 2012-01-27 | Lung Neoplasms; Carcinoma, Adenoid Cystic; Prostatic Neoplasms, Castration-Resistant; Breast Neoplasms; Sarcoma; Prostatic Neoplasms; Cholangiocarcinoma; Hepatic Insufficiency; Colorectal Neoplasms; Thyroid Neoplasms; Neuroendocrine Tumors; Leukemia, Myeloid, Acute; Carcinoma, Pancreatic Ductal; Lymphoma; Carcinoma, Hepatocellular; Adenocarcinoma; Carcinoma, Non-Small-Cell Lung; Melanoma; Paraganglioma; Myelodysplastic Syndromes; Ovarian Neoplasms; Solid tumours; Carcinoma, Renal Cell; Carcinoma; Pheochromocytoma; Squamous Cell Carcinoma of Head and Neck; Stomach Neoplasms; Carcinoid Tumor; Kidney Neoplasms; Neoplasms; Nasopharyngeal Neoplasms; Adrenal Cortex Neoplasms; Skin Neoplasms; Pancreatic Neoplasms; Glioblastoma; Mesothelioma; Carcinoma, Ductal | Details |
| Regorafenib | DAST; BAY-73-4506 | Approved | Bayer AG | Stivarga, Resihance | United States | Colorectal Neoplasms | Bayer Healthcare Pharmaceuticals Inc | 2012-09-27 | Fallopian Tube Neoplasms; Osteosarcoma; Sarcoma, Ewing; Colorectal Neoplasms; Gastrointestinal Stromal Tumors; Peritoneal Neoplasms; Bone Neoplasms; Bile Duct Neoplasms; Thymoma; Thyroid Neoplasms; Leukemia, Myeloid, Acute; Gastrinoma; Lung Neoplasms; Esophageal adenocarcinoma; Carcinoma, Hepatocellular; Carcinoma, Non-Small-Cell Lung; Melanoma; Gastrointestinal Neoplasms; Somatostatinoma; Adenocarcinoma; Neoplasm Metastasis; Meningioma; Neoplasms; Solid tumours; Ovarian Neoplasms; Rectal Neoplasms; Carcinoma, Renal Cell; Hemangiosarcoma; Carcinoid Tumor; Insulinoma; Carcinoma, Islet Cell; Stomach Neoplasms; Esophageal Neoplasms; Liver Neoplasms; Carcinoma, Transitional Cell; Colonic Neoplasms; Pancreatic Neoplasms; Glioblastoma; Carcinoma, Ovarian Epithelial; Adenoma; Glucagonoma; Carcinoma, Adenoid Cystic; Sarcoma | Details |
| Nintedanib Esylate | BIBF-1120 | Approved | C.H. Boehringer Sohn Ag & Co. Kg | Ofev, Vargatef | United States | Idiopathic Pulmonary Fibrosis | Boehringer Ingelheim Gmbh | 2014-10-15 | Lung Neoplasms; Breast Neoplasms; Prostatic Neoplasms; Adenocarcinoma, Clear Cell; systemic sclerosis-associated interstitial lung disease; Colorectal Neoplasms; Peritoneal Neoplasms; Gliosarcoma; Hepatic Insufficiency; Astrocytoma; Genital Neoplasms, Female; Silicosis; Sarcoma; Carcinoma, Squamous Cell; Fallopian Tube Neoplasms; Appendiceal Neoplasms; Leukemia, Myeloid, Acute; Uterine Neoplasms; Endometrial Neoplasms; Carcinoma, Non-Small-Cell Lung; Carcinoma, Hepatocellular; Adenocarcinoma; Carcinoma, Endometrioid; Scleroderma, Systemic; Telangiectasia, Hereditary Hemorrhagic; Solid tumours; Rejection of lung transplantation; Carcinoma, Renal Cell; Radiation Pneumonitis; Esophageal Neoplasms; Carcinoid Tumor; Endometrial Stromal Tumors; Idiopathic Pulmonary Fibrosis; Neoplasms; Ovarian Neoplasms; Glioblastoma; Colonic Neoplasms; Small Cell Lung Carcinoma; Pulmonary Fibrosis; Lung Diseases, Interstitial; Oligodendroglioma; Multiple Myeloma; Mesothelioma; Asbestosis; Neuroendocrine Tumors | Details |
| Vorolanib | X-82; EYP-1901; CM-082 | Approved | Tyrogenex Inc | 伏美纳 | Mainland China | Carcinoma, Renal Cell | Betta Pharmaceuticals Co Ltd | 2023-06-07 | Diabetic macular oedema; Carcinoma, Non-Small-Cell Lung; Melanoma; Macular Degeneration; Carcinoma, Hepatocellular; Diabetic Retinopathy; Choroidal Neovascularization; Adenocarcinoma; Retinal Degeneration; Leukemia, Myeloid, Acute; Eye Diseases; Solid tumours; Wet Macular Degeneration; Retinal Diseases; Small Cell Lung Carcinoma; Neoplasms; Pancreatic Neoplasms; Stomach Neoplasms; Esophageal Neoplasms; Carcinoma, Renal Cell; Kidney Neoplasms | Details |
| Tivozanib | Kil-8951; AV-951; KRN-951; ASP-4130; KHK-4951; KHK4951; UNII-172030934T | Approved | Kyowa Hakko Kirin Co Ltd | Fotivda, FOTIVDA | EU | Carcinoma, Renal Cell | Recordati Netherlands BV | 2017-08-24 | Colorectal Neoplasms; Carcinoma, Hepatocellular; Carcinoma, Non-Small-Cell Lung; Gastrointestinal Neoplasms; Macular Degeneration; Fallopian Tube Neoplasms; Metastatic breast cancer; Peritoneal Neoplasms; Hepatic Insufficiency; Bile Duct Neoplasms; Biliary Tract Neoplasms; Sarcoma; Breast Neoplasms; Cholangiocarcinoma; Carcinoma, Ovarian Epithelial; Triple Negative Breast Neoplasms; Carcinoma, Renal Cell; Ovarian Neoplasms; Liver Neoplasms; Solid tumours | Details |
| Apatinib Mesylate | YN-968D1 | Approved | Advenchen Laboratories Llc | 艾坦, Aitan | Mainland China | Stomach Neoplasms | Jiangsu shengdi Medicine Co Ltd | 2014-10-17 | Nasopharyngeal Neoplasms; Adenocarcinoma; Carcinoma, Hepatocellular; Carcinoma, Non-Small-Cell Lung; Lung Neoplasms; Fallopian Tube Neoplasms; Colorectal Neoplasms; Peritoneal Neoplasms; Nasopharyngeal Carcinoma; Breast Neoplasms; Carcinoma, Adenoid Cystic; Head and Neck Neoplasms; Triple Negative Breast Neoplasms; Carcinoma, Ovarian Epithelial; Neoplasms; Esthesioneuroblastoma, Olfactory; Esophageal Neoplasms; Stomach Neoplasms; Solid tumours; Ovarian Neoplasms; Liver Neoplasms; Biliary Tract Neoplasms | Details |
| Ripretinib | DCC-2618 | Approved | Deciphera | Qinlock, 擎乐 | United States | Gastrointestinal Stromal Tumors | Deciphera Pharmaceuticals Llc | 2020-05-15 | Neoplasms; Mastocytosis, Systemic; Gastrointestinal Stromal Tumors | Details |
| Ramucirumab | IMC-1121; IMC-1C11; LY-3009806; IMC-1121-B; BLA-125477 | Approved | Dyax Pharma | Cyramza | United States | Gastrointestinal Stromal Tumors | Eli Lilly And Company | 2014-04-21 | Breast Neoplasms; Melanoma; Carcinoma, Hepatocellular; Adenocarcinoma; Carcinoma, Non-Small-Cell Lung; Fallopian Tube Neoplasms; Ureteral Neoplasms; Urologic Neoplasms; Peritoneal Neoplasms; Gastrointestinal Stromal Tumors; Colorectal Neoplasms; Prostatic Neoplasms; Urethral Neoplasms; Liver Neoplasms; Pancreatic Neoplasms; Colonic Neoplasms; Carcinoma, Transitional Cell; Neoplasms; Carcinoma, Renal Cell; Rectal Neoplasms; Esophageal Neoplasms; Carcinoid Tumor; Stomach Neoplasms; Biliary Tract Neoplasms; Ovarian Neoplasms; Solid tumours | Details |
| Sunitinib Malate | PNU-290940AD; PHA-290940AD; SCAI-003; GB-102; PNU-290940; SU-011248-L-malate salt; PHA-290940; SU-010398; SU-11248 | Approved | Pfizer Inc | 索坦, Sutent | United States | Carcinoma, Renal Cell; Gastrointestinal Stromal Tumors | Cppi Cv | 2006-01-26 | Solid tumours; Fibromatosis, Aggressive; Ovarian Neoplasms; Kidney Neoplasms; Leukemia, Myelogenous, Chronic; Head and Neck Neoplasms; Leiomyosarcoma; Lymphoma, B-Cell; Lymphoma, B-Cell, Marginal Zone; HIV Infections; Leukemia, Myeloid, Accelerated Phase; Leukemia; Fibrosarcoma; Teratoma; Liver Neoplasms; Ependymoma; Lymphoma, T-Cell, Peripheral; Intestinal Neoplasms; Histiocytoma, Malignant Fibrous; Carcinoma, Renal Cell; Hemangioblastoma; Carcinoma, Islet Cell; Carcinoma; Pheochromocytoma; Stomach Neoplasms; Pelvic Neoplasms; Esophageal Neoplasms; Leukemia, Hairy Cell; Polycythemia Vera; Abdominal Neoplasms; Squamous Cell Carcinoma of Head and Neck; Thoracic Neoplasms; Pancreatic neuroendocrine tumors (pNET); Carcinoma, Ovarian Epithelial; Glioblastoma; Neurofibromatoses; Small Cell Lung Carcinoma; Adenoma, Islet Cell; Carcinoma, Transitional Cell; Pancreatic Neoplasms; Lymphoma, Large B-Cell, Diffuse; Neoplasms; Myelodysplastic Syndromes; Hodgkin Disease; Leukemia, Myelomonocytic, Chronic; Carcinoma, Papil | Details |
| Pazopanib Hydrochloride | GSK-786034; GW-786034B; SB-786034; GW-786034 | Approved | Glaxosmithkline Plc, Novartis Pharma Ag | 维全特, Armala, Votrient, Patorma | United States | Carcinoma, Renal Cell; Sarcoma | Novartis Pharma Ag | 2009-10-19 | Uterine Cervical Diseases; Fallopian Tube Neoplasms; Lung Neoplasms; Uterine Neoplasms; Choriocarcinoma; Lymphoma; Colorectal Neoplasms; Gastrointestinal Stromal Tumors; Carcinoma, Mucoepidermoid; Gliosarcoma; Genital Neoplasms, Female; Leukemia, Myeloid, Acute; Peritoneal Neoplasms; Brain Neoplasms; Urethral Neoplasms; Medullary thyroid cancer (MTC); Chondrosarcoma, Extraskeletal Myxoid; Prostatic Neoplasms; Osteosarcoma; Neuroblastoma; Sarcoma; Breast Neoplasms; Carcinoma, Hepatocellular; Uterine Cervical Neoplasms; Breast Neoplasms, Male; Neoplasm Metastasis; von Hippel-Lindau Disease; Thyroid Cancer, Papillary; Gastrointestinal Neoplasms; Paraganglioma; Endodermal Sinus Tumor; Neoplasms, Germ Cell and Embryonal; Melanoma; Gastrinoma; Macular Degeneration; Carcinoma, Non-Small-Cell Lung; Thyroid Neoplasms; Carcinoma, Squamous Cell; Carcinoma, Small Cell; Carcinoma, Embryonal; Germinoma; Glioma; Carcinoma, Neuroendocrine; Epistaxis; Carcinoma, Ovarian Epithelial; Neoplasms; Squamous Cell Carcinoma of Head a | Details |
| Cabozantinib S-malate | XL-184; BMS-907351 | Approved | Exelixis Inc | Cometriq, Cabometyx | United States | Carcinoma, Neuroendocrine; Thyroid Neoplasms | Exelixis Inc | 2012-11-29 | Urethral Neoplasms; Leukemia, Myeloid, Acute; Thyroid Neoplasms; Gliosarcoma; Colorectal Neoplasms; Astrocytoma; Hepatic Insufficiency; Peritoneal Neoplasms; Bile Duct Neoplasms; Sarcoma, Clear Cell; Uterine Neoplasms; Adenocarcinoma, Clear Cell; Sarcoma, Ewing; Carcinoma, Adenosquamous; Breast Neoplasms; Neurofibroma, Plexiform; Osteosarcoma; Prostatic Neoplasms; Sarcoma; Medullary thyroid cancer (MTC); Carcinoma, Hepatocellular; Melanoma; Paraganglioma; Meningioma; Neoplasms, Germ Cell and Embryonal; Neoplasm Metastasis; Carcinoma, Non-Small-Cell Lung; Adenocarcinoma; Carcinoma, Endometrioid; Thyroid Cancer, Papillary; Brain Neoplasms; Carcinoma, Neuroendocrine; Sarcoma, Alveolar Soft Part; Lymphoma; Fallopian Tube Neoplasms; Brain metastases; Glioma; Endometrial Neoplasms; Carcinoma, Squamous Cell; Carcinoid Tumor; Glioblastoma; Skin Neoplasms; Neoplasms; Carcinoma, Papillary; Hepatoblastoma; Pheochromocytoma; Pain; Rejection of liver transplantation; Carcinoma, Renal Cell; Pancreatic neuroendocrine tumors | Details |
| Fruquintinib | HMPL-013; TAK-113 | Approved | Hutchison Medipharma Ltd | Elunate, FRUZAQLA, Aiyoute, 爱优特 | Mainland China | Colorectal Neoplasms | Hutchison Medipharma Ltd | 2018-09-04 | Small Cell Lung Carcinoma; Carcinoma, Non-Small-Cell Lung; Endometrial Neoplasms; Lung Neoplasms; Hepatic Insufficiency; Colorectal Neoplasms; Breast Neoplasms; Sarcoma; Kidney Diseases; Neoplasms; Solid tumours; Triple Negative Breast Neoplasms; Pancreatic Neoplasms; Colonic Neoplasms; Rectal Neoplasms; Esophageal Neoplasms; Stomach Neoplasms; Carcinoma, Renal Cell; Biliary Tract Neoplasms | Details |
| Sorafenib Tosylate | NSC-724772; BAY-43-0006; BAY-43-9006; BAY-54-9085 | Approved | Onyx Pharmaceuticals Inc | Nexavar, 多吉美 | United States | Carcinoma, Renal Cell | Bayer Healthcare Pharmaceuticals Inc | 2005-12-01 | Liver Neoplasms; Kidney Neoplasms; Recurrence; Ovarian Neoplasms; Leukemia, Myelogenous, Chronic; Lymphoma, T-Cell, Peripheral; Leukemia, Erythroblastic, Acute; Rhabdomyosarcoma; Lymphoma, B-Cell, Marginal Zone; Fibromatosis, Aggressive; Head and Neck Neoplasms; Solid tumours; Leiomyosarcoma; Leukemia, Myeloid; Carcinoma, Renal Cell; Carcinoma; Vipoma; Esophageal Neoplasms; Hemangiosarcoma; Squamous Cell Carcinoma of Head and Neck; Rectal Neoplasms; Carcinoid Tumor; Histiocytoma, Malignant Fibrous; Carcinoma, Islet Cell; Insulinoma; Stomach Neoplasms; Neoplasms; Kidney Diseases; Small Cell Lung Carcinoma; Carcinoma, Transitional Cell; Carcinoma, Verrucous; Thyroid Carcinoma, Anaplastic; Myelodysplastic Syndromes; Glioblastoma; Pancreatic Neoplasms; Leukemia, Myelomonocytic, Chronic; Carcinoma, Ovarian Epithelial; Leukemia, Myelomonocytic, Acute; Wilms Tumor; Lymphomatoid Granulomatosis; Colonic Neoplasms; Hypertension, Portal; Lymphoma, Large B-Cell, Diffuse; Lymphoma, Large-Cell, Immunoblastic; Multiple Endo | Details |
| Vandetanib | AZD-6474; CH-331; ZD-6474 | Approved | Astrazeneca Plc | Zactima, Caprelsa | United States | Carcinoma, Neuroendocrine; Thyroid Neoplasms | Genzyme Corp | 2011-04-06 | Lung Neoplasms; Neuroblastoma; Medullary thyroid cancer (MTC); Prostatic Neoplasms; Urethral Neoplasms; Peritoneal Neoplasms; Gliosarcoma; Colorectal Neoplasms; Ureteral Neoplasms; Gastrointestinal Stromal Tumors; Diffuse Intrinsic Pontine Glioma; Lymphoproliferative Disorders; Astrocytoma; Thyroid Neoplasms; Pleural Effusion; Carcinoma, Squamous Cell; Lymphoma; Fallopian Tube Neoplasms; Multiple Endocrine Neoplasia Type 2b; Glioma; Carcinoma, Neuroendocrine; Carcinoma, Non-Small-Cell Lung; Paraganglioma; Precancerous Conditions; Neoplasm Metastasis; Adenocarcinoma; von Hippel-Lindau Disease; Carcinoma, Hepatocellular; Anus Neoplasms; Biliary Tract Neoplasms; Solid tumours; Kidney Neoplasms; Intestinal Neoplasms; Ovarian Neoplasms; Pheochromocytoma; Stomach Neoplasms; Esophageal Neoplasms; Carcinoma, Renal Cell; Carcinoma; Squamous Cell Carcinoma of Head and Neck; Rectal Neoplasms; Abdominal Neoplasms; Head and Neck Neoplasms; Small Cell Lung Carcinoma; Carcinoma, Transitional Cell; Pancreatic Neoplasms; Neop | Details |
| Midostaurin | PKC-412; PKC-412A; CGP-41231; CGP-41251 | Approved | Novartis Pharma Ag | Rydapt | United States | Mastocytosis, Systemic; Leukemia, Mast-Cell; Hematologic Neoplasms; Leukemia, Myeloid, Acute | Novartis Pharmaceuticals Corp | 2017-04-28 | Leukemia; Hematologic Neoplasms; Mastocytosis, Systemic; Myelodysplastic Syndromes; Leukemia, Mast-Cell; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Hepatic Insufficiency; Leukemia, Myeloid, Acute; Mastocytosis | Details |
| English Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| Zanzalintinib | XL-092 | Phase 3 Clinical | Exelixis Inc | Solid tumours; Carcinoma, Renal Cell; Squamous Cell Carcinoma of Head and Neck; Carcinoma, Transitional Cell; Neoplasms; Prostatic Neoplasms, Castration-Resistant; Breast Neoplasms; Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung; Carcinoma, Hepatocellular | Details |
| JY-025 | JY-025 | Phase 3 Clinical | Beijing Jingyitaixiang Technology Development Co Ltd, Beijing Eastern Biotech Co Ltd | Liver Neoplasms; Solid tumours; Stomach Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| Recombinant anti-VEGFR2 chimeric monoclonaly antibody (GeneScience) | Phase 3 Clinical | Changchun GeneScience Pharmaceuticals Co Ltd | Solid tumours; Esophageal Neoplasms; Stomach Neoplasms; Gastrointestinal Neoplasms; Carcinoma, Non-Small-Cell Lung | Details | |
| Lucitanib | S-80881; AL-3810; CO-3810; E-3810; S-80881-2 | Phase 3 Clinical | Advenchen Laboratories Nanjing Ltd | Solid tumours; Stomach Neoplasms; Small Cell Lung Carcinoma; Nasopharyngeal Carcinoma; Breast Neoplasms; Colorectal Neoplasms; Genital Neoplasms, Female; Thymus Neoplasms; Lung Neoplasms; Carcinoma, Small Cell; Carcinoma, Hepatocellular; Carcinoma, Non-Small-Cell Lung | Details |
| Recombinant human anti-VEGFR2 monoclonal antibody (Buchang Pharma) | BC-001; BC001 | Phase 3 Clinical | Shandong Buchang Pharmaceuticals Co Ltd | Solid tumours; Esophageal Neoplasms; Colorectal Neoplasms | Details |
| KC1036 | KC1036; KC-1036 | Phase 3 Clinical | Beijing Konruns Pharmaceutical Co Ltd | Hematologic Neoplasms; Solid tumours; Digestive System Neoplasms; Sarcoma, Ewing; Thymus Neoplasms; Esophageal Squamous Cell Carcinoma; Adenocarcinoma; Neoplasm Metastasis | Details |
| Cediranib | AZD2171; AZD-2171; NSC-732208 | Phase 3 Clinical | Astrazeneca Plc | Solid tumours; Ovarian Neoplasms; Head and Neck Neoplasms; Biliary Tract Neoplasms; Leiomyosarcoma; Medulloblastoma; Leukemia; Ependymoma; Lymphoma, T-Cell, Peripheral; Lymphoma, B-Cell; Lymphoma, B-Cell, Marginal Zone; Liver Neoplasms; Carcinoma; Rhabdoid Tumor; Leukemia, Hairy Cell; Squamous Cell Carcinoma of Head and Neck; Rectal Neoplasms; Carcinoma, Renal Cell; Stomach Neoplasms; Cystadenocarcinoma, Serous; Spinal Cord Neoplasms; Cystadenoma, Serous; Cystadenocarcinoma, Mucinous; Glioblastoma; Pancreatic Neoplasms; Carcinoma, Verrucous; Neoplasms; Nasopharyngeal Neoplasms; Myelodysplastic Syndromes; Carcinoma, Ovarian Epithelial; Hodgkin Disease; Hypopharyngeal Neoplasms; Colonic Neoplasms; Salivary Gland Neoplasms; Lymphomatoid Granulomatosis; Lymphoma, Large-Cell, Immunoblastic; Lymphoma, Large B-Cell, Diffuse; Small Cell Lung Carcinoma; Leukemia-Lymphoma, Adult T-Cell; Carcinoma, Transitional Cell; Triple Negative Breast Neoplasms; Oligodendroglioma; Uveal melanoma; Adenocarcinoma, Follicular; Neuroen | Details |
| Sitravatinib | MG-91516; MGCD-516; IND-155305; MG-516 | Phase 3 Clinical | Mirati Therapeutics Inc | Breast Neoplasms; Carcinoma, Hepatocellular; Gastrointestinal Neoplasms; Uterine Cervical Neoplasms; Carcinoma, Non-Small-Cell Lung; Metastatic breast cancer; Mouth Neoplasms; Endometrial Neoplasms; Carcinoma, Squamous Cell; Esophageal Squamous Cell Carcinoma; Lung Neoplasms; Ureteral Neoplasms; Hepatic Insufficiency; Solid tumours; Liposarcoma; Uveal melanoma; Lung Diseases; Carcinoma, Transitional Cell; Neoplasms; Triple Negative Breast Neoplasms; Carcinoma; Squamous Cell Carcinoma of Head and Neck; Carcinoma, Renal Cell; Biliary Tract Neoplasms; Kidney Neoplasms | Details |
| Gentuximab | Phase 3 Clinical | Genescience Pharmaceuticals Co Ltd | Adenocarcinoma; Carcinoma, Non-Small-Cell Lung | Details | |
| Axitinib intravitreal implant (Ocular Therapeutix) | OTX-TKI | Phase 3 Clinical | Ocular Therapeutix Inc | Wet Macular Degeneration; Macular Degeneration; Diabetic Retinopathy | Details |
| Pulocimab | AK-109; AK109 | Phase 3 Clinical | Kang Rong Dongfang (Guangdong) Pharmaceutical Co Ltd | Solid tumours; Esophageal Neoplasms; Stomach Neoplasms; Adenocarcinoma | Details |
| Ningetinib Tosylate | CT-053-PTSA; CT-053 | Phase 2 Clinical | Hec Pharm Co Ltd | Intestinal Neoplasms; Solid tumours; Carcinoma, Renal Cell; Stomach Neoplasms; Leukemia, Myeloid, Acute; Lung Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| Simmitinib hydrochloride | SOMCL-15-290 | Phase 2 Clinical | Shanghai Institute Of Materia Medica, Chinese Academy Of Sciences | Solid tumours; Neoplasms; Esophageal Squamous Cell Carcinoma | Details |
| AUR-109 | ODM-203; AUR-109 | Phase 2 Clinical | Orion Corp | Ovarian Neoplasms; Liver Neoplasms; Solid tumours; Carcinoma, Renal Cell; Urinary Bladder Neoplasms; Pulmonary Fibrosis; Breast Neoplasms; Lung Neoplasms | Details |
| VEGFR2 peptide vaccine (VAXIMM) | VXM-01 | Phase 2 Clinical | Merck Serono | Glioblastoma; Pancreatic Neoplasms; Colorectal Neoplasms | Details |
| Telatinib | EOC-315; BAY-57-9352 | Phase 2 Clinical | Bayer AG | Solid tumours; Stomach Neoplasms | Details |
| Brivanib Alaninate | ZL-2301; BMS-540215; BMS-582664 | Phase 2 Clinical | Bristol-Myers Squibb Company | Carcinoma, Squamous Cell; Carcinoma, Endometrioid; Adenocarcinoma; Uterine Cervical Neoplasms; Neoplasm Metastasis; Gastrointestinal Neoplasms; Carcinoma, Non-Small-Cell Lung; Carcinoma, Hepatocellular; Endometrial Neoplasms; Liver Neoplasms; Colorectal Neoplasms; Sarcoma; Neoplasms; Pancreatic Neoplasms; Carcinoma, Transitional Cell; Carcinoma, Renal Cell; Rectal Neoplasms; Solid tumours | Details |
| Derazantinib | ARQ-087.2HCl; AQ-14741087; ARQ-087; BAL-087 | Phase 2 Clinical | Arqule Inc | Biliary Tract Neoplasms; Solid tumours; Stomach Neoplasms; Carcinoma, Transitional Cell; Cholangiocarcinoma; Urogenital Neoplasms; Carcinoma, Hepatocellular | Details |
| Glesatinib | MGCD-265; MG-90265X; 7Q29OXD98N; MG-90265H9; MG-90265gly; MG-90265 | Phase 2 Clinical | Mirati Therapeutics Inc | Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| Olinvacimab | SSS-23; TTAC-0001 | Phase 2 Clinical | Pharmabcine | Neoplasms; Glioblastoma; Triple Negative Breast Neoplasms; Neoplasm Metastasis | Details |
| BR-55 | BR-55; BR55 CEUS | Phase 2 Clinical | Sonus Pharmaceuticals Ltd | Ovarian Neoplasms; Neoplasms; Contrast agents; Diagnostic agents; Inflammation | Details |
| Axitinib Implant(Aerie) | AR-14034 SR; AR-14034 | Phase 2 Clinical | Aerie Pharmaceuticals Inc | Macular Degeneration | Details |
| Sorafenib Tosylate/Comekibart | MG-D-1609 | Phase 2 Clinical | Metagone Biotech Inc | Solid tumours | Details |
| VEGFR-1/2 peptide vaccine (Keio University) | Phase 2 Clinical | Keio University | Neurofibromatoses; Brain Neoplasms; Glioma | Details | |
| Gersizangitide | AXT-107 | Phase 2 Clinical | AsclepiX Therapeutics Inc | Macular Edema; Diabetic macular oedema; Macular Degeneration | Details |
| KDR2-2 | Phase 2 Clinical | Guangzhou Huiborui Biomedical Technology Co Ltd | Neovascularization, Pathologic; Glaucoma, Neovascular; Corneal Neovascularization | Details | |
| KHK-4951 | Phase 2 Clinical | Kyowa Kirin | Macular Edema; Wet Macular Degeneration; Diabetic macular oedema; Macular Degeneration | Details | |
| PAN-90806 | CP-632; OSI-632; CP-547632; PAN-90806 | Phase 2 Clinical | Pfizer Inc, Osi Pharmaceutical | Ovarian Neoplasms; Abdominal Neoplasms; Peritoneal Neoplasms; Fallopian Tube Neoplasms; Lung Neoplasms; Macular Degeneration; Diabetic Retinopathy | Details |
| CEP-11981 | ESK-981; CEP-11981; SSR-106462; BOL-303213X | Phase 2 Clinical | Sanofi | Adenoma, Islet Cell; Pancreatic Neoplasms; Neoplasms; Prostatic Neoplasms, Castration-Resistant; Prostatic Neoplasms; Carcinoma, Adenosquamous; Carcinoma, Neuroendocrine; Gastrointestinal Neoplasms | Details |
| Axitinib injectable suspension (Clearside Biomedical) | CLS-1002; CLS-011-A; CLS011A; CLS-AX | Phase 2 Clinical | Clearside Biomedical Inc | Macular Degeneration | Details |
| MSB-0254 | MSB-0254 | Phase 1 Clinical | Mabspace Biomedicine (Suzhou) Co Ltd | Solid tumours | Details |
| EDP317 | ACTB-1003; EOC-317; EDP-317 | Phase 1 Clinical | Bayer AG, Act Biotech Inc | Biliary Tract Neoplasms; Solid tumours; Stomach Neoplasms; Urinary Bladder Neoplasms; Breast Neoplasms | Details |
| Kanitinib | CX-1003 | Phase 1 Clinical | Beijing Konruns Pharmaceutical Co Ltd, Guangzhou Yingsheng Bio Technology Co Ltd | Solid tumours | Details |
| VEGFR2 peptide vaccine (Tokyo University) | Phase 1 Clinical | University Of Tokyo | Pancreatic Neoplasms | Details | |
| Ramucirumab biosimilar (Henlius) | HLX-12 | Phase 1 Clinical | Shanghai Henlius Biotech Inc | Stomach Neoplasms; Esophageal Neoplasms; Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| AVI-3207 | AVI-3207 | Phase 1 Clinical | Avixgen | Macular Degeneration | Details |
| Pamufetinib | TAS-115 | Phase 1 Clinical | Taiho Pharmaceutical Co Ltd, Otsuka Pharmaceutical Co Ltd | Neoplasms | Details |
| Ramucirumab biosimilar (Sichuan Kelunbotai Biopharmaceutical) | Phase 1 Clinical | Sichuan Kelun Pharmaceutical Co Ltd | Solid tumours; Stomach Neoplasms; Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung | Details | |
| Tafetinib Malate | SIM-0702; SIM-1005; SIM-010603 | Phase 1 Clinical | Jiangsu Simcere Pharmaceutical Co Ltd, Nanjing Yoko Biomedical Co Ltd, Jilin Boda Pharmaceutical Co Ltd | Neoplasms | Details |
| QBH-196 | QBH-196 | Phase 1 Clinical | Shenyang Pharmaceutical University | Solid tumours; Stomach Neoplasms | Details |
| ETN-101 | ETN101; ETN-101; MBP-11901 | Phase 1 Clinical | Etnova Therapeutics Corp | Liver Neoplasms; Carcinoma, Hepatocellular | Details |
| RA1115-B1 | RA1115-B1 | Phase 1 Clinical | Suzhou Raymon Pharmaceuticals Co Ltd | Wet Macular Degeneration; Macular Degeneration | Details |
| PZ-1 | PZ-1 | Phase 1 Clinical | Chongqing University Of Arts And Sciences | Solid tumours | Details |
| SYHA-1817 | SYHA-1817 | Phase 1 Clinical | CSPC Pharmaceutical Group Ltd | Biliary Tract Neoplasms; Stomach Neoplasms; Carcinoma, Squamous Cell | Details |
| Ramucirumab biosimilar (CTTQ) | Phase 1 Clinical | Jiangsu Chia Tai-Tianqing Pharmaceutical Co Ltd | Stomach Neoplasms; Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung | Details | |
| LMV-12(HE003) | LMV-12(HE003) | Phase 1 Clinical | Nanchang Hongyi Technology Co Ltd | Solid tumours | Details |
| Metatinib Trometamol | BMS-817378; SIM-817378 | Phase 1 Clinical | Bristol-Myers Squibb Company | Solid tumours; Liver Neoplasms; Stomach Neoplasms; Colorectal Neoplasms | Details |
| NP-01 (Shijiazhuang No.4 Pharmaceutical/Nanjing Nadingfei Medical Technology) | NP-01 | Phase 1 Clinical | Shijiazhuang No 4 Pharmaceutical Co Ltd, Nanjing Nadingfei Pharmaceutical Technology Co Ltd | Solid tumours; Liver Neoplasms; Stomach Neoplasms; Lung Neoplasms | Details |
| FNX-006 | FNX-006 | Phase 1 Clinical | Sichuan University, Chengdu Fannuoxi Biomedical Technology Co Ltd | Triple Negative Breast Neoplasms; Melanoma | Details |
| Zanzalintinib | XL-092 | Phase 3 Clinical | Exelixis Inc | Solid tumours; Carcinoma, Renal Cell; Squamous Cell Carcinoma of Head and Neck; Carcinoma, Transitional Cell; Neoplasms; Prostatic Neoplasms, Castration-Resistant; Breast Neoplasms; Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung; Carcinoma, Hepatocellular | Details |
| JY-025 | JY-025 | Phase 3 Clinical | Beijing Jingyitaixiang Technology Development Co Ltd, Beijing Eastern Biotech Co Ltd | Liver Neoplasms; Solid tumours; Stomach Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| Recombinant anti-VEGFR2 chimeric monoclonaly antibody (GeneScience) | Phase 3 Clinical | Changchun GeneScience Pharmaceuticals Co Ltd | Solid tumours; Esophageal Neoplasms; Stomach Neoplasms; Gastrointestinal Neoplasms; Carcinoma, Non-Small-Cell Lung | Details | |
| Lucitanib | S-80881; AL-3810; CO-3810; E-3810; S-80881-2 | Phase 3 Clinical | Advenchen Laboratories Nanjing Ltd | Solid tumours; Stomach Neoplasms; Small Cell Lung Carcinoma; Nasopharyngeal Carcinoma; Breast Neoplasms; Colorectal Neoplasms; Genital Neoplasms, Female; Thymus Neoplasms; Lung Neoplasms; Carcinoma, Small Cell; Carcinoma, Hepatocellular; Carcinoma, Non-Small-Cell Lung | Details |
| Recombinant human anti-VEGFR2 monoclonal antibody (Buchang Pharma) | BC-001; BC001 | Phase 3 Clinical | Shandong Buchang Pharmaceuticals Co Ltd | Solid tumours; Esophageal Neoplasms; Colorectal Neoplasms | Details |
| KC1036 | KC1036; KC-1036 | Phase 3 Clinical | Beijing Konruns Pharmaceutical Co Ltd | Hematologic Neoplasms; Solid tumours; Digestive System Neoplasms; Sarcoma, Ewing; Thymus Neoplasms; Esophageal Squamous Cell Carcinoma; Adenocarcinoma; Neoplasm Metastasis | Details |
| Cediranib | AZD2171; AZD-2171; NSC-732208 | Phase 3 Clinical | Astrazeneca Plc | Solid tumours; Ovarian Neoplasms; Head and Neck Neoplasms; Biliary Tract Neoplasms; Leiomyosarcoma; Medulloblastoma; Leukemia; Ependymoma; Lymphoma, T-Cell, Peripheral; Lymphoma, B-Cell; Lymphoma, B-Cell, Marginal Zone; Liver Neoplasms; Carcinoma; Rhabdoid Tumor; Leukemia, Hairy Cell; Squamous Cell Carcinoma of Head and Neck; Rectal Neoplasms; Carcinoma, Renal Cell; Stomach Neoplasms; Cystadenocarcinoma, Serous; Spinal Cord Neoplasms; Cystadenoma, Serous; Cystadenocarcinoma, Mucinous; Glioblastoma; Pancreatic Neoplasms; Carcinoma, Verrucous; Neoplasms; Nasopharyngeal Neoplasms; Myelodysplastic Syndromes; Carcinoma, Ovarian Epithelial; Hodgkin Disease; Hypopharyngeal Neoplasms; Colonic Neoplasms; Salivary Gland Neoplasms; Lymphomatoid Granulomatosis; Lymphoma, Large-Cell, Immunoblastic; Lymphoma, Large B-Cell, Diffuse; Small Cell Lung Carcinoma; Leukemia-Lymphoma, Adult T-Cell; Carcinoma, Transitional Cell; Triple Negative Breast Neoplasms; Oligodendroglioma; Uveal melanoma; Adenocarcinoma, Follicular; Neuroen | Details |
| Sitravatinib | MG-91516; MGCD-516; IND-155305; MG-516 | Phase 3 Clinical | Mirati Therapeutics Inc | Breast Neoplasms; Carcinoma, Hepatocellular; Gastrointestinal Neoplasms; Uterine Cervical Neoplasms; Carcinoma, Non-Small-Cell Lung; Metastatic breast cancer; Mouth Neoplasms; Endometrial Neoplasms; Carcinoma, Squamous Cell; Esophageal Squamous Cell Carcinoma; Lung Neoplasms; Ureteral Neoplasms; Hepatic Insufficiency; Solid tumours; Liposarcoma; Uveal melanoma; Lung Diseases; Carcinoma, Transitional Cell; Neoplasms; Triple Negative Breast Neoplasms; Carcinoma; Squamous Cell Carcinoma of Head and Neck; Carcinoma, Renal Cell; Biliary Tract Neoplasms; Kidney Neoplasms | Details |
| Gentuximab | Phase 3 Clinical | Genescience Pharmaceuticals Co Ltd | Adenocarcinoma; Carcinoma, Non-Small-Cell Lung | Details | |
| Axitinib intravitreal implant (Ocular Therapeutix) | OTX-TKI | Phase 3 Clinical | Ocular Therapeutix Inc | Wet Macular Degeneration; Macular Degeneration; Diabetic Retinopathy | Details |
| Pulocimab | AK-109; AK109 | Phase 3 Clinical | Kang Rong Dongfang (Guangdong) Pharmaceutical Co Ltd | Solid tumours; Esophageal Neoplasms; Stomach Neoplasms; Adenocarcinoma | Details |
| Ningetinib Tosylate | CT-053-PTSA; CT-053 | Phase 2 Clinical | Hec Pharm Co Ltd | Intestinal Neoplasms; Solid tumours; Carcinoma, Renal Cell; Stomach Neoplasms; Leukemia, Myeloid, Acute; Lung Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| Simmitinib hydrochloride | SOMCL-15-290 | Phase 2 Clinical | Shanghai Institute Of Materia Medica, Chinese Academy Of Sciences | Solid tumours; Neoplasms; Esophageal Squamous Cell Carcinoma | Details |
| AUR-109 | ODM-203; AUR-109 | Phase 2 Clinical | Orion Corp | Ovarian Neoplasms; Liver Neoplasms; Solid tumours; Carcinoma, Renal Cell; Urinary Bladder Neoplasms; Pulmonary Fibrosis; Breast Neoplasms; Lung Neoplasms | Details |
| VEGFR2 peptide vaccine (VAXIMM) | VXM-01 | Phase 2 Clinical | Merck Serono | Glioblastoma; Pancreatic Neoplasms; Colorectal Neoplasms | Details |
| Telatinib | EOC-315; BAY-57-9352 | Phase 2 Clinical | Bayer AG | Solid tumours; Stomach Neoplasms | Details |
| Brivanib Alaninate | ZL-2301; BMS-540215; BMS-582664 | Phase 2 Clinical | Bristol-Myers Squibb Company | Carcinoma, Squamous Cell; Carcinoma, Endometrioid; Adenocarcinoma; Uterine Cervical Neoplasms; Neoplasm Metastasis; Gastrointestinal Neoplasms; Carcinoma, Non-Small-Cell Lung; Carcinoma, Hepatocellular; Endometrial Neoplasms; Liver Neoplasms; Colorectal Neoplasms; Sarcoma; Neoplasms; Pancreatic Neoplasms; Carcinoma, Transitional Cell; Carcinoma, Renal Cell; Rectal Neoplasms; Solid tumours | Details |
| Derazantinib | ARQ-087.2HCl; AQ-14741087; ARQ-087; BAL-087 | Phase 2 Clinical | Arqule Inc | Biliary Tract Neoplasms; Solid tumours; Stomach Neoplasms; Carcinoma, Transitional Cell; Cholangiocarcinoma; Urogenital Neoplasms; Carcinoma, Hepatocellular | Details |
| Glesatinib | MGCD-265; MG-90265X; 7Q29OXD98N; MG-90265H9; MG-90265gly; MG-90265 | Phase 2 Clinical | Mirati Therapeutics Inc | Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| Olinvacimab | SSS-23; TTAC-0001 | Phase 2 Clinical | Pharmabcine | Neoplasms; Glioblastoma; Triple Negative Breast Neoplasms; Neoplasm Metastasis | Details |
| BR-55 | BR-55; BR55 CEUS | Phase 2 Clinical | Sonus Pharmaceuticals Ltd | Ovarian Neoplasms; Neoplasms; Contrast agents; Diagnostic agents; Inflammation | Details |
| Axitinib Implant(Aerie) | AR-14034 SR; AR-14034 | Phase 2 Clinical | Aerie Pharmaceuticals Inc | Macular Degeneration | Details |
| Sorafenib Tosylate/Comekibart | MG-D-1609 | Phase 2 Clinical | Metagone Biotech Inc | Solid tumours | Details |
| VEGFR-1/2 peptide vaccine (Keio University) | Phase 2 Clinical | Keio University | Neurofibromatoses; Brain Neoplasms; Glioma | Details | |
| Gersizangitide | AXT-107 | Phase 2 Clinical | AsclepiX Therapeutics Inc | Macular Edema; Diabetic macular oedema; Macular Degeneration | Details |
| KDR2-2 | Phase 2 Clinical | Guangzhou Huiborui Biomedical Technology Co Ltd | Neovascularization, Pathologic; Glaucoma, Neovascular; Corneal Neovascularization | Details | |
| KHK-4951 | Phase 2 Clinical | Kyowa Kirin | Macular Edema; Wet Macular Degeneration; Diabetic macular oedema; Macular Degeneration | Details | |
| PAN-90806 | CP-632; OSI-632; CP-547632; PAN-90806 | Phase 2 Clinical | Pfizer Inc, Osi Pharmaceutical | Ovarian Neoplasms; Abdominal Neoplasms; Peritoneal Neoplasms; Fallopian Tube Neoplasms; Lung Neoplasms; Macular Degeneration; Diabetic Retinopathy | Details |
| CEP-11981 | ESK-981; CEP-11981; SSR-106462; BOL-303213X | Phase 2 Clinical | Sanofi | Adenoma, Islet Cell; Pancreatic Neoplasms; Neoplasms; Prostatic Neoplasms, Castration-Resistant; Prostatic Neoplasms; Carcinoma, Adenosquamous; Carcinoma, Neuroendocrine; Gastrointestinal Neoplasms | Details |
| Axitinib injectable suspension (Clearside Biomedical) | CLS-1002; CLS-011-A; CLS011A; CLS-AX | Phase 2 Clinical | Clearside Biomedical Inc | Macular Degeneration | Details |
| MSB-0254 | MSB-0254 | Phase 1 Clinical | Mabspace Biomedicine (Suzhou) Co Ltd | Solid tumours | Details |
| EDP317 | ACTB-1003; EOC-317; EDP-317 | Phase 1 Clinical | Bayer AG, Act Biotech Inc | Biliary Tract Neoplasms; Solid tumours; Stomach Neoplasms; Urinary Bladder Neoplasms; Breast Neoplasms | Details |
| Kanitinib | CX-1003 | Phase 1 Clinical | Beijing Konruns Pharmaceutical Co Ltd, Guangzhou Yingsheng Bio Technology Co Ltd | Solid tumours | Details |
| VEGFR2 peptide vaccine (Tokyo University) | Phase 1 Clinical | University Of Tokyo | Pancreatic Neoplasms | Details | |
| Ramucirumab biosimilar (Henlius) | HLX-12 | Phase 1 Clinical | Shanghai Henlius Biotech Inc | Stomach Neoplasms; Esophageal Neoplasms; Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| AVI-3207 | AVI-3207 | Phase 1 Clinical | Avixgen | Macular Degeneration | Details |
| Pamufetinib | TAS-115 | Phase 1 Clinical | Taiho Pharmaceutical Co Ltd, Otsuka Pharmaceutical Co Ltd | Neoplasms | Details |
| Ramucirumab biosimilar (Sichuan Kelunbotai Biopharmaceutical) | Phase 1 Clinical | Sichuan Kelun Pharmaceutical Co Ltd | Solid tumours; Stomach Neoplasms; Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung | Details | |
| Tafetinib Malate | SIM-0702; SIM-1005; SIM-010603 | Phase 1 Clinical | Jiangsu Simcere Pharmaceutical Co Ltd, Nanjing Yoko Biomedical Co Ltd, Jilin Boda Pharmaceutical Co Ltd | Neoplasms | Details |
| QBH-196 | QBH-196 | Phase 1 Clinical | Shenyang Pharmaceutical University | Solid tumours; Stomach Neoplasms | Details |
| ETN-101 | ETN101; ETN-101; MBP-11901 | Phase 1 Clinical | Etnova Therapeutics Corp | Liver Neoplasms; Carcinoma, Hepatocellular | Details |
| RA1115-B1 | RA1115-B1 | Phase 1 Clinical | Suzhou Raymon Pharmaceuticals Co Ltd | Wet Macular Degeneration; Macular Degeneration | Details |
| PZ-1 | PZ-1 | Phase 1 Clinical | Chongqing University Of Arts And Sciences | Solid tumours | Details |
| SYHA-1817 | SYHA-1817 | Phase 1 Clinical | CSPC Pharmaceutical Group Ltd | Biliary Tract Neoplasms; Stomach Neoplasms; Carcinoma, Squamous Cell | Details |
| Ramucirumab biosimilar (CTTQ) | Phase 1 Clinical | Jiangsu Chia Tai-Tianqing Pharmaceutical Co Ltd | Stomach Neoplasms; Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung | Details | |
| LMV-12(HE003) | LMV-12(HE003) | Phase 1 Clinical | Nanchang Hongyi Technology Co Ltd | Solid tumours | Details |
| Metatinib Trometamol | BMS-817378; SIM-817378 | Phase 1 Clinical | Bristol-Myers Squibb Company | Solid tumours; Liver Neoplasms; Stomach Neoplasms; Colorectal Neoplasms | Details |
| NP-01 (Shijiazhuang No.4 Pharmaceutical/Nanjing Nadingfei Medical Technology) | NP-01 | Phase 1 Clinical | Shijiazhuang No 4 Pharmaceutical Co Ltd, Nanjing Nadingfei Pharmaceutical Technology Co Ltd | Solid tumours; Liver Neoplasms; Stomach Neoplasms; Lung Neoplasms | Details |
| FNX-006 | FNX-006 | Phase 1 Clinical | Sichuan University, Chengdu Fannuoxi Biomedical Technology Co Ltd | Triple Negative Breast Neoplasms; Melanoma | Details |
This web search service is supported by Google Inc.





