



























 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!
 Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!  Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!

This protein carries a human IgG1 Fc tag at the C-terminus
The protein has a calculated MW of 92.7 kDa. The protein migrates as 100-120 kDa under reducing (R) condition (SDS-PAGE) due to glycosylation.
>95% as determined by SDS-PAGE.
Lyophilized from 0.22 μm filtered solution in Tris with Glycine, Arginine and NaCl, pH7.5 with trehalose as protectant.
Contact us for customized product form or formulation.
Please see Certificate of Analysis for specific instructions.
For best performance, we strongly recommend you to follow the reconstitution protocol provided in the CoA.
For long term storage, the product should be stored at lyophilized state at -20°C or lower.
Please avoid repeated freeze-thaw cycles.
This product is stable after storage at:
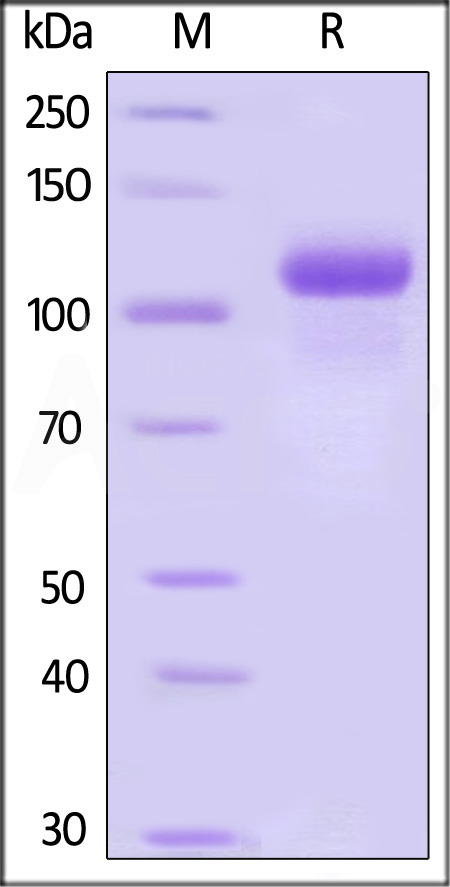
Human G-CSF R, Fc Tag on SDS-PAGE under reducing (R) condition. The gel was stained with Coomassie Blue. The purity of the protein is greater than 95%.

Immobilized Human G-CSF Protein, premium grade (Cat. No. GCF-H5214) at 2 μg/mL (100 μL/well) can bind Human G-CSF R, Fc Tag (Cat. No. GCR-H5250) with a linear range of 1-16 ng/mL (QC tested).

ACROBiosystems는 생체 활성이 높은 균질한 CD3 δ/CD3 ε 및 CD3 γ/CD3 ε 단백질을 개발하여 이중특이 항체를 치료제로의 임상 개발을 가속시킵니다.

We offer a wide range of cell and gene therapy solutions starting from discovery to the clinic. Explore our wide range of proteins, antibodies, kits, and other assays to accelerate the development of your cell and gene therapy.

오가노이드 툴 박스는 약물 개발 프로젝트의 진행을 가속할 수 있는 레디메이드 오가노이드와 오가노이드 분화 툴 키트 및 다양한 서비스를 포함하는 오가노이드 솔루션 모음입니다.

고품질 GMP 등급 사이토카인 IL-15,IL-7, IL-21 등 제품은 신속한 임상/신약승인신청을 위해 개발된 것입니다.

50+ car-t의 인기 타겟을 커버하고, car 발현 검사를 위해 특별히 설계된 항원 단백질은 스트림 검증을 통해 고특이성 car 발현을 검출할 수 있으며, 고 배치간 일관성을 가지고 있다.pe/fi표기 단백질로 한 단계 염색하면 고특이성, 무 백그라운드로 car 발현 검사를 검사할 수 있습니다.

cd20, claudin18.2, cd133, gprc5d, ccr5, ccr8을 포함한 안정적이고 활성이 높은 전장 다중 막관통 단백질은 면역, elisa, spr, bli, 세포 실험, car 양성률 검사 등에 광범위하게 사용된다. vlp, 스케일제거제, nanodisc의 다양한 기술 플랫폼은 다중 막관통 단백질을 목표로 하는 약물 연구에 도움을 준다.

GMP 등급 사이토카인. 고품질 세포 활성화 및 확장 시약. 유전자 편집 시약, 효소 CAR 검사 시약 등 제품. 세포 및 유전자 요법 고객님에게 포괄적인 솔루션을 제공하고 초기 약물 발견에서 임상 연구에 이르기까지 모든 단계에서 귀하와 동반하겠습니다.

현재 이미 알려진 거의 대부분의 면역 체크 포인트 분자를 포함하여 다양한 레벨과 물종을 제공하여 고객이 선택할수 있도록 하고있다. 천연중합체 형식은 mals를 통해 검증되었고 생물활성은 elisa/spr/bli/facs 등을 통해 검증되었으며 또한 biotin/fitc 표지는 높은 처리량을 가진 항체를 선별하는데 편리하다.

ACROBiosystems는 ADC약물의 연구개발을 돕기 위해 노력하고 있으며, 다양한 인기 타겟에 서로 다른 종, 다른 라벨의 제품을 개발했으며, 높은 순도, 높은 친화력 등 특징을 가지고 있으며, 면역, 항체 선택, spr, 세포 활성 검사 및 기타 실험에 적합하고, 해당 protocol을 무료로 제공합니다.

Fc 수용체 단백질은 모든 분자를 포함하고 있을 뿐만 아니라, 일반적인 돌연변이와 바이오틴 표기 종류을 포함하고 있어 고객들이 단일 클론 항체 개발을 가속화할 수 있다.

포괄적으로 풍부한 사이토카인 표적 단백질은 HEK293 진핵생물 시스템에 의해 발현되며 천연 구조에 더 가깝다. 고순도는 SDS-PAGE/HPLC/MALS에 의해 검증된다. 높은 생물학적 활성이 ELISA/SPR/BLI에 의해 검증된다. 배치간 일관성이 우수하여 항체 면역, 스크리닝 및 품질 관리 과정에 적합하다.

Aneuro는 ACROBiosystems가 뇌과학 연구에 초점을 맞춘 제품 라인으로서 치료 및 진단 연구 단백질, PFFs와 재조합 신경 인자 등 뇌과학 연구 분야에 중요한 단백질 제품을 제공하여 뇌과학 연구에 도움이 된다.
| English Name | Research Code | Research Phase | Company | First Brand Name | First Approved Country | First Indication | First Approved Company | First Approved Date | Indications | Clinical Trials |
|---|---|---|---|---|---|---|---|---|---|---|
| Pegfilgrastim | R-1471; SD-01; SD/01; KRN-125 | Approved | Kyowa Hakko Kirin Co Ltd, Amgen Inc | Ristempa, Neulasta, G-Lasta, Neulastim | United States | Neutropenia | Amgen Inc | 2002-01-31 | Urinary Bladder Neoplasms; Adenocarcinoma; Melanoma; Carcinoma, Non-Small-Cell Lung; Endometrial Neoplasms; Leukemia, Myeloid, Acute; Lung Neoplasms; Lymphoma; Lymphoma, Non-Hodgkin; Colorectal Neoplasms; Prostatic Neoplasms; Carcinoma, Adenosquamous; Sarcoma; Neurofibrosarcoma; Sarcoma, Ewing; Breast Neoplasms; Diabetes Mellitus, Type 1; Sarcoma, Synovial; Multiple Myeloma; Colonic Neoplasms; Small Cell Lung Carcinoma; Pancreatic Neoplasms; Carcinoma; Rectal Neoplasms; Stomach Neoplasms; Esophageal Neoplasms; Neutropenia; Ovarian Neoplasms; Solid tumours; Rhabdomyosarcoma; Head and Neck Neoplasms | Details |
| Empegfilgrastim | BCD-017 | Approved | Biocad | Extimia | Neutropenia | Details | ||||
| Pegfilgrastim biosimilar (Coherus BioSciences) | CHS-1701 | Approved | Coherus Biosciences Inc | Udenyca, UDENYCA | United States | Febrile Neutropenia | Coherus Biosciences Inc | 2018-09-21 | Neutropenia; Acute Radiation Syndrome; Febrile Neutropenia | Details |
| Filgrastim biosimilar (Institute of Medical Biology Chinese Academy of Medical Sciences) | Approved | Institute Of Medical Biology, Chinese Academy Of Medical Sciences | 吉洛因 | Mainland China | Myelodysplastic Syndromes; Leukopenia | Institute Of Medical Biology, Chinese Academy Of Medical Sciences | 2001-01-01 | Myelodysplastic Syndromes; Leukopenia | Details | |
| Pegfilgrastim biosimilar (Hospira) | HSP-130 | Approved | Pfizer Inc, Hospira | Nyvepria | United States | Febrile Neutropenia | Hospira Inc | 2020-06-10 | Neutropenia; Lymphoma, Large B-Cell, Diffuse; Lymphoma, AIDS-Related; Breast Neoplasms; Febrile Neutropenia | Details |
| Nartograstim | KW-2228; PE-2601; ND-28 | Approved | Kyowa Hakko Kirin Co Ltd | Neu-up | Japan | Neutropenia | Kyowa Hakko Kirin Co Ltd | 1994-04-01 | Neutropenia | Details |
| Pegfilgrastim biosimilar (Juta Pharma) | Approved | Juta Pharma | Grasustek | EU | Neutropenia | Juta Pharma Gmbh | 2019-04-26 | Neutropenia | Details | |
| Filgrastim biosimilar (Nanogen) | Approved | Nanogen Biopharmaceutical Co | Ficocyte | Vietnam | Neutropenia | Nanogen Biopharmaceutical Co | 2014-01-01 | Neutropenia | Details | |
| Filgrastim biosimilar (Blau Pharmaceuticals) | Approved | Blau Pharmaceuticals | Brazil | Neutropenia | Blau Pharmaceuticals | 2012-10-01 | Neutropenia | Details | ||
| Filgrastim biosimilar (Accord Healthcare) | Approved | Accord Healthcare | Accofil | EU | Neutropenia | Accord Healthcare Slu | 2014-09-17 | Neutropenia | Details | |
| Filgrastim biosimilar (PanPharmaceuticals) | Approved | Harvest Moon | India | Febrile Neutropenia | null | 2012-01-01 | Febrile Neutropenia | Details | ||
| Filgrastim biosimilar (Biosidus) | Approved | Biosidus | Neutromax, Granulostim | Neutropenia | Details | |||||
| Filgrastim biosimilar (Biocon) | Approved | Biocon Biopharmaceuticals | Nufil | India | Neutropenia | null | 2008-01-01 | Neutropenia | Details | |
| Pegfilgrastim biosimilar (Beijing SL Pharmaceutical) | Approved | Beijing Sl Pharmaceutical Co Ltd | Mainland China | Neutropenia | Beijing Sl Pharmaceutical Co Ltd | 2023-09-19 | Neutropenia | Details | ||
| Filgrastim biosimilar (Unilab) | Approved | Unilab | Neocyte | Neutropenia | Details | |||||
| Filgrastim biosimilar (GeneScience Pharmaceuticals) | Approved | Changchun GeneScience Pharmaceuticals Co Ltd | Scimax, 金磊赛强 | Mainland China | Neutropenia | Changchun GeneScience Pharmaceuticals Co Ltd | 1998-01-01 | Neutropenia | Details | |
| Pegfilgrastim biosimilar (Zydus Cadila) | Approved | Zydus Cadila | India | Neutropenia | Zydus Cadila | 2014-01-01 | Neutropenia | Details | ||
| Filgrastim biosimilar (Veropharm) | Approved | Abbott Laboratories | Mielastra | Neutropenia | Details | |||||
| Filgrastim | rhGCSF (Amgen/Kyowa Hakko Kirin); r-metHuG-CSF; REC-G-CSF; KRN-8601 | Approved | Amgen Inc, Kyowa Hakko Kirin Co Ltd | 吉粒芬, Gran, Nugraf, Neupogen, Granulokine, 惠尔血, 优保津, 特尔津, 瑞白, 吉赛欣, 津恤力 | United States | Neutropenia | Amgen Inc | 1991-02-20 | Leukemia, Myeloid, Chronic-Phase; Critical Illness; Malnutrition; Pancytopenia; Anemia, Aplastic; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Musculoskeletal Diseases; Fever; Primary Myelofibrosis; Colorectal Neoplasms; Alzheimer Disease; Breast Neoplasms; Sickle Cell Trait; Lymphoma; Ischemia; Leg Ulcer; Leukemia, Myeloid, Acute; Thrombocytopenia; Leukemia, Myelomonocytic, Juvenile; Melanoma; Crohn Disease; Respiratory Insufficiency; Cardiomyopathy, Dilated; Solid tumours; Leukemia, Myelogenous, Chronic; HIV Infections; Diabetic Foot; Neutropenia; Menopausal symptoms; Hot Flashes; Myelodysplastic Syndromes; Graft vs Host Disease; Myeloproliferative Disorders; Leukemia; Liver Cirrhosis; Anemia, Diamond-Blackfan; Pancreatic Neoplasms; Fanconi Anemia; Dyskeratosis Congenita; Stroke; Multiple Myeloma; Uveal melanoma; Shwachman-Diamond Syndrome; Neuroblastoma | Details |
| Filgrastim biosimilar (Apotex/Intas) | Approved | Apotex, Intas Biopharmaceuticals | Grastofil, SciLocyte, Neukine | India | Neutropenia | null | 2004-01-01 | Neutropenia | Details | |
| Pegfilgrastim biosimilar (Harvest Moon Pharmaceuticals/PanPharmaceuticals) | Approved | Harvest Moon | India | Neutropenia | null | 2012-01-01 | Neutropenia | Details | ||
| Filgrastim biosimilar (Pharmstandard/Masterclone/CJSC Generium) | Approved | Cjsc Generium, Pharmstandard, Masterclone | Neipomax, Neupomax | Neutropenia | null | 2008-01-01 | Neutropenia | Details | ||
| Pegfilgrastim biosimilar (Intas Biopharmaceuticals) | INTP-5 | Approved | Intas Biopharmaceuticals, Apotex | Neupeg, Lapelga, Pegasta | India | Neutropenia | null | 2007-01-01 | Hypospadias; Neutropenia; Multiple Myeloma; Lymphoma; Febrile Neutropenia | Details |
| Filgrastim biosimilar (PT Kalbe) | Approved | Kalbe Farma | India | Neutropenia | Kalbe Farma | 2012-08-01 | Neutropenia | Details | ||
| Filgrastim biosimilar (Claris Lifesciences) | Approved | Claris Lifesciences Ltd | Fegrast | India | Neutropenia | Claris Lifesciences Ltd | 2012-01-01 | Neutropenia | Details | |
| Efbemalenograstim alfa | F-627 | Approved | Evive (Shanghai) Corp Ltd | Bineuta, 亿立舒, Ryzneuta | Mainland China | Neutropenia | Evive Biopharmaceutical (Beijing) Co Ltd | 2023-05-06 | Ovarian Neoplasms; Neutropenia; Neoplasms; Breast Neoplasms; Fever; Febrile Neutropenia; Carcinoma, Non-Small-Cell Lung; Uterine Cervical Neoplasms | Details |
| Recombinant human granulocyte colony-stimulating factor (Shandong Kexing Bioproducts Co Ltd) | Approved | Shandong Kexing Bio-Products Co Ltd | 白特喜 | Mainland China | Neutropenia | Shandong Kexing Bio-Products Co Ltd | 2001-01-01 | Neutropenia | Details | |
| Filgrastim biosimilar (Apexcela) | SBS-6002; Leuco-Plus-300 | Approved | Apexcela | Neutropenia | Details | |||||
| Recombinant human granulocyte colony-stimulating Factor (Shenzhen Xinpeng Bio-tech) | Approved | Shenzhen Sinobioway Xinpeng Biomedicine Co Ltd | 瑞血新 | Mainland China | Neutropenia | Shenzhen Sinobioway Xinpeng Biomedicine Co Ltd | 1999-01-01 | Neutropenia | Details | |
| Filgrastim biosimilar (CJ Corp) | Approved | HK inno.N Corporation | Neutropenia | Details | ||||||
| Filgrastim biosimilar (Xiamen Amoytop Biotech) | Approved | Xiamen Amoytop Biotech Co Ltd | 特尔津, Topneuter | Mainland China | Neutropenia | Xiamen Amoytop Biotech Co Ltd | 1999-01-01 | Neutropenia | Details | |
| Filgrastim biosimilar (Eurofarma) | Approved | Eurofarma | Granulen | Brazil | Neutropenia | Eurofarma | 2012-01-01 | Neutropenia | Details | |
| Filgrastim biosimilar (Sedico) | Approved | Sedico | Neutropenia | Details | ||||||
| Filgrastim biosimilar (Zenotech Laboratories) | Approved | Zenotech Laboratories | Neoplasms; Neutropenia | Zenotech Laboratories | 2006-03-01 | Neutropenia; Neoplasms | Details | |||
| Filgrastim biosimilar (Lupin) | Approved | Lupin Ltd | Lupifil | India | Neutropenia | Lupin Ltd | 2016-01-01 | Neutropenia | Details | |
| Filgrastim biosimilar (Beijing SL Pharmaceutical) | Approved | Beijing Sl Pharmaceutical Co Ltd | 立生素, Li Sheng Su | Mainland China | Neutropenia | Beijing Sl Pharmaceutical Co Ltd | 1998-01-01 | Neutropenia | Details | |
| Pegfilgrastim biosimilar (Nanogen Biopharmaceutical) | Approved | Nanogen Biopharmaceutical Co | Neutropenia | Details | ||||||
| Filgrastim biosimilar (Biolotus Biotech) | Approved | Biolotus | Kymun-CSF | Brazil | Neutropenia | Biolotus | 2016-06-01 | Neutropenia | Details | |
| Filgrastim biosimilar (Fresenius Kabi Oncology) | Approved | Fresenius Kabi Ag | India | Neutropenia | Fresenius Kabi Ag | 2012-08-01 | Neutropenia | Details | ||
| Tbo-filgrastim | TKN-732; XM-02 | Approved | Teva | Tevagrastim, Biograstim, Granix | United States | Neutropenia | Sicor Biotech Uab | 2008-09-15 | Neutropenia; Multiple Myeloma; Lymphoma, Non-Hodgkin | Details |
| Filgrastim biosimilar (Laboratorios AC Farma) | Approved | Laboratorios Ac Farma | FGM-Factor | Peru | Neutropenia | Laboratorios Ac Farma | 2012-01-01 | Neutropenia | Details | |
| Filgrastim biosimilar(Pooyesh Darou Pharmaceuticals) | G-CSF | Approved | Pooyesh Darou | PD-grastim | Iran | Myelodysplastic Syndromes; Leukemia, Myeloid, Acute; Lymphoma; Neutropenia | Pooyesh Darou | 2012-09-01 | Neutropenia; Myelodysplastic Syndromes; Leukemia, Myeloid, Acute; Lymphoma | Details |
| Filgrastim biosimilar (Chandra Bhagat Pharma) | Approved | Chandra Bhagat Pharma(Cbc) | Filgen | India | Neoplasms | Chandra Bhagat Pharma(Cbc) | 2012-08-01 | Neoplasms | Details | |
| Filgrastim biosimilar (Zydus Cadila) | Approved | Zydus Cadila | India | Neutropenia | Zydus Cadila | 2014-01-01 | Neutropenia | Details | ||
| Filgrastim biosimilar (AqVida) | Approved | Aqvida Gmbh | Leucita | Russian Federation | Neutropenia | Aqvida Gmbh | 2008-05-01 | Neutropenia | Details | |
| Filgrastim biosimilar (Incepta Pharmaceuticals) | Approved | Incepta Pharmaceuticals Ltd | Neutropenia | Details | ||||||
| Pegfilgrastim biosimilar (AryaTinaGene) | Approved | Aryatinagene Biopharmaceuticals | Tinapeg | Febrile Neutropenia | Details | |||||
| Recombinant human granulocyte colony-stimulating factor (Shanghai Sunway Biotech) | Approved | Shanghai Sunway Bio Co Ltd | 赛格力, SunGran | Mainland China | Neutropenia | Shanghai Sunway Bio Co Ltd | 1998-01-01 | Neutropenia | Details | |
| Filgrastim biosimilar (Laboratorio Varifarma) | Approved | Laboratorio Varifarma | Neutrofil | Neutropenia | Details | |||||
| Recombinant human granulocyte colony-stimulating factor (Harbin Pharmaceutical Group Bioengineering) | Approved | Harbin Pharmaceutical Group Holding Co Ltd | 里亚金, Li Ya Jin | Mainland China | Neutropenia | Harbin Pharmaceutical Group Holding Co Ltd | 2000-01-01 | Neutropenia | Details | |
| Eflapegrastim | LAPS-GCSF; HNK-460; SPI-2012; HM-10460A | Approved | Hanmi Pharmaceutical Co Ltd | ROLVEDON, Rolontis, Rolvedon, ROLONTIS | South Korea | Neutropenia | Hanmi Pharmaceutical Co Ltd | 2021-03-18 | Solid tumours; Neutropenia; Neoplasms; Breast Neoplasms; Lymphoma; Febrile Neutropenia | Details |
| Recombinant Human Granulocyte Colony-stimulating Factor (Wuzhong Group) | Approved | Suzhou Pharmaceutical Factory | 洁欣, Jiexin | Mainland China | Neutropenia | Suzhou Pharmaceutical Factory | 1999-01-01 | Neutropenia | Details | |
| Filgrastim biosimilar (Chalver Laboratorios) | Approved | Chalver Laboratorios | Leucosos | Colombia | Neutropenia | Chalver Laboratorios | 2012-01-01 | Neutropenia | Details | |
| Filgrastim biosimilar (Siam Bioscience) | Approved | Siam Bioscience | Neutropenia | Details | ||||||
| Filgrastim biosimilar (Reliance Life Sciences) | Approved | Reliance Life Sciences | ReliGrast | India | Neutropenia | Reliance Life Sciences | 2008-01-01 | Neutropenia | Details | |
| Filgrastim biosimilar (Tanvex BioPharma) | TX-01 | Approved | Tanvex Biopharma | Canada | Febrile Neutropenia | Tanvex Biopharma | 2021-10-17 | Febrile Neutropenia | Details | |
| Recombinant human granulocyte stimulating factor (CSPC Pharma) | Approved | CSPC Pharmaceutical Group Ltd | 津恤力, GeneLeukim | Mainland China | Neutropenia | Cspc Baike (Shandong) Biopharmaceutical Co Ltd | 2000-01-01 | Neutropenia | Details | |
| Filgrastim biosimilar (Dong-A) | DA-3030 | Approved | Dong-A Pharmaceutical Co Ltd | Leukostim | South Korea | Neutropenia | Dong-A Pharmaceutical Co Ltd | 1999-01-01 | Drug-Related Side Effects and Adverse Reactions; Neutropenia; Diabetic Neuropathies; Breast Neoplasms | Details |
| Filgrastim biosimilar (Adello Biologics/KASHIV BIOSCIENCES) | Approved | Adello Biologics Llc | United States | Neutropenia; Febrile Neutropenia; Chemotherapy-Induced Febrile Neutropenia | Kashiv BioSciences LLC | 2022-02-25 | Chemotherapy-Induced Febrile Neutropenia; Neutropenia; Febrile Neutropenia | Details | ||
| Pegfilgrastim biosimilar (Amega Biotech) | Approved | Amega Biotech | Neutropenia | Details | ||||||
| Filgrastim biosimilar (Hangzhou Jiuyuan Gene Engineering) | Approved | Hangzhou Jiuyuan Gene Engineering Co Ltd | 吉粒芬, Jilifen | Mainland China | Neutropenia | Hangzhou Jiuyuan Gene Engineering Co Ltd | 1998-04-01 | Neutropenia | Details | |
| Filgrastim biosimilar (Probiomed) | Approved | Probiomed | Filatil | Mexico | Neutropenia | Probiomed | 2006-03-01 | Neutropenia | Details | |
| Filgrastim biosimilar (Emcure Pharmaceuticals) | Approved | Emcure Pharma | India | Neutropenia | Emcure Pharma | 2013-01-01 | Neutropenia | Details | ||
| Mecapegfilgrastim (Hengrui) | HHPG-19K | Approved | Jiangsu Hengrui Medicine Co Ltd | 艾多 | Mainland China | Neutropenia | Jiangsu Hengrui Medicine Co Ltd | 2018-05-08 | Neutropenia; Neoplasms; Breast Neoplasms; Febrile Neutropenia; Carcinoma, Non-Small-Cell Lung | Details |
| Filgrastim biosimilar (Hospira) | PLD-108 | Approved | Hospira | Nivestim, Nivestym | EU | Neutropenia; Neoplasms; Hematopoietic stem cell transplantation (HSCT) | Pfizer Europe Ma Eeig | 2010-06-07 | Hematopoietic stem cell transplantation (HSCT); Neutropenia; Neoplasms; Febrile Neutropenia | Details |
| Recombinant human granulocyte colony stimulating Factor (Beijing Four Rings Bio-Pharmaceutical) | Approved | Beijing Four Rings Bio-Pharmaceutical Co Ltd | 欣粒生, Xin Lisheng | Mainland China | Neutropenia | Beijing Four Rings Bio-Pharmaceutical Co Ltd | 2002-06-17 | Neutropenia | Details | |
| Pegfilgrastim biosimilar (Fresenius SE) | MSB-11455 | Approved | Merck Serono | Stimufend | EU | Neutropenia; Chemotherapy-Induced Febrile Neutropenia | Fresenius Kabi Deutschland Gmbh | 2022-03-28 | Chemotherapy-Induced Febrile Neutropenia; Neutropenia; Febrile Neutropenia | Details |
| Filgrastim biosimilar (Zuventus Healthcare) | Approved | Zuventus Healthcare | India | Neutropenia | Zuventus Healthcare | 2012-08-01 | Neutropenia | Details | ||
| Lenograstim biosimilar (Biosidus) | Approved | Biosidus | Lenobio, Leumostin, Lenograstim Biosidus | Neutropenia | Details | |||||
| Lipegfilgrastim | XM-22 | Approved | Teva | Lonquex | EU | Neutropenia | Teva Bv | 2013-07-25 | Rhabdomyosarcoma; Neutropenia; Multiple Myeloma; Lymphoma | Details |
| Filgrastim biosimilar (Neiss Labs) | Approved | Neiss Labs | Nisgras | India | Neutropenia | Neiss Labs | 2012-08-01 | Neutropenia | Details | |
| Filgrastim biosimilar (Fuji Pharmaceutical/Mochida/Gene Techno Science) | GBS-001; FSK-0808 | Approved | Mochida Pharmaceutical Co Ltd, Gene Techno Science, Fuji Pharmaceutical | Japan | Neutropenia | Fuji Pharmaceutical | 2012-11-21 | Neutropenia | Details | |
| Filgrastim biosimilar (Center of Molecular Immunology) | Approved | Center Of Molecular Immunology | ior-LeukoCIM | Cuba | Neutropenia | Center Of Molecular Immunology | 2016-01-14 | Neutropenia | Details | |
| Lenograstim | rG-CSF | Approved | Chugai Pharmaceutical Co Ltd | 格拉诺赛特, Neutrogin, Granocyte | Japan | Neutropenia | Chugai Pharmaceutical Co Ltd | 1991-10-04 | Neutropenia; Hodgkin Disease; Multiple Myeloma; Lymphoma, Non-Hodgkin | Details |
| Pegfilgrastim biosimilar (CinnaGen) | Approved | Cinnagen | PegaGen | Neutropenia | Details | |||||
| Pegfilgrastim biosimilar (Emcure Pharmaceuticals) | Approved | Emcure Pharma | Neutropenia | Details | ||||||
| GCPGC | MG-1107 | Approved | GC Biopharma Corp | Neulapeg | South Korea | Neutropenia | GC Biopharma Corp | 2014-01-01 | Neutropenia; Pancreatic Neoplasms | Details |
| Filgrastim biosimilar (RAS Lifesciences) | Approved | Ras Lifesciences | India | Neutropenia | Ras Lifesciences | 2012-01-01 | Neutropenia | Details | ||
| Filgrastim biosimilar (Sudershan Biotech) | Approved | Sudershan Biotech Ltd | Neutropenia | Details | ||||||
| Filgrastim biosimilar (Shandong Quangang Pharmaceutical) | Approved | Shandong Quangang Pharmaceutical Co Ltd | 泉升, Quan Sheng | Mainland China | Neutropenia | Shandong Quangang Pharmaceutical Co Ltd | 2002-04-16 | Neutropenia | Details | |
| Pegfilgrastim biosimilar (Qilu Pharmaceuticals) | PEG-rhG-CSF (Qilu Pharmaceuticals) | Approved | Qilu Pharmaceutical Co Ltd | 新瑞白 | Mainland China | Neutropenia | Qilu Pharmaceutical Co Ltd | 2015-08-08 | Neutropenia | Details |
| Filgrastim biosimilar (RPG Life Sciences) | Approved | Rpg Life Sciences Ltd | Frastim | India | Neutropenia | Rpg Life Sciences Ltd | 2012-08-01 | Neutropenia | Details | |
| Pegfilgrastim biosimilar (Sandoz) | LAEP-2006 | Approved | Clariant Produkte (Schweiz) Ag | Zioxtenzo, Ziextenzo | EU | Neutropenia | Sandoz Gmbh | 2018-11-22 | Drug-Related Side Effects and Adverse Reactions; Neutropenia; Breast Neoplasms; Febrile Neutropenia | Details |
| Telpegfilgrastim | YPEG-rhG-CSF | Approved | Xiamen Amoytop Biotech Co Ltd | 珮金 | Mainland China | Febrile Neutropenia | Xiamen Amoytop Biotech Co Ltd | 2023-06-30 | Neutropenia; Neoplasms; Breast Neoplasms; Carcinoma, Non-Small-Cell Lung; Growth hormone deficiency; Febrile Neutropenia | Details |
| Filgrastim biosimilar (Landsteiner) | Approved | Landsteiner Scientific | Biofilgran | Neutropenia | Details | |||||
| Filgrastim biosimilar (Sandoz) | EP-2006 | Approved | Sandoz | Zarxio, Zarzio | EU | Neutropenia | Hexal Ag | 2009-02-06 | Neutropenia; Breast Neoplasms; Febrile Neutropenia | Details |
| Pegfilgrastim biosimilar (Cinfa Biotech) | B-12019 | Approved | Cinfa Biotech Gmbh | Pelmeg | EU | Neutropenia | Mundipharma Corporation (Ireland) Ltd | 2018-11-20 | Neutropenia | Details |
| Filgrastim biosimilar (Pharmapark) | Approved | Pharmapark | Neutropenia | Pharmapark | 2008-01-01 | Neutropenia | Details | |||
| Filgrastim biosimilar (NCPC GeneTech Biotechnology Development) | Approved | North China Pharmaceutical Company Ltd | GeSysin | Mainland China | Neutropenia | North China Pharmaceutical Company Ltd | 1999-01-01 | Neutropenia | Details | |
| Filgrastim biosimilar (Chengdu Institute of Biological Products) | Approved | Chengdu Institute Of Biological Products Co Ltd | 保力津, Baolijin | Mainland China | Neutropenia | Chengdu Institute Of Biological Products Co Ltd | 2001-01-01 | Neutropenia | Details | |
| Pegfilgrastim biosimilar (mochida) | GBS-010; MD110 | Approved | Mochida Pharmaceutical Co Ltd | Japan | Febrile Neutropenia | Mochida Pharmaceutical Co Ltd | 2023-09-25 | Chemotherapy-Induced Febrile Neutropenia; Febrile Neutropenia | Details | |
| Recombinant Human Granulocyte/Macrophage Colony-stimulating Factor for Injection (Foshan Hanyu) | Approved | Foshan Hanyu Biopharmaceutical Co Ltd | 格宁 | Mainland China | Neutropenia | Foshan Hanyu Biopharmaceutical Co Ltd | 1998-01-01 | Neutropenia | Details | |
| Recombinant Human Granulocyte/Macrophage Colony-stimulating Factor for Injection (Beijing Beiyi Union) | Approved | Beijing Beiyi Union Pharmaceutical Co Ltd | 健白 | Mainland China | Neutropenia | Beijing Beiyi Union Pharmaceutical Co Ltd | 2002-05-08 | Neutropenia | Details | |
| Recombinant human granulocyte macrophage colony stimulating factor (Kyowa Hakko Kirin) | Approved | Kyowa Kirin Co Ltd, Ise Plant, Nipro Pharma Corp, Kyowa Kirin China Pharmaceutical Co Ltd | Details | |||||||
| Pegfilgrastim biosimilar(Shandong New Time Pharmaceutical ) | Approved | Shandong New Time Pharmaceutical Co Ltd | Details | |||||||
| Pegfilgrastim biosimilar (Biocon/Mylan) | MYL-1401H | Approved | Biocon Ltd | Fulphila | United States | Febrile Neutropenia | Mylan Gmbh | 2018-06-04 | Chemotherapy-Induced Febrile Neutropenia; Neutropenia; Breast Neoplasms; Febrile Neutropenia | Details |
| Pegfilgrastim biosimilar (Dong-A ST) | DA-3031 | Approved | Dong-A Pharmaceutical Co Ltd | Dulastin | South Korea | Neutropenia | Dong-A Pharmaceutical Co Ltd | 2014-01-01 | Solid tumours; Neutropenia; Lymphoma | Details |
| Filgrastim biosimilar (CIGB/Heber Biotec) | Approved | Center For Genetic Engineering And Biotechnology, Heber Biotec | Hebervital | Neutropenia | null | 2009-01-01 | Neutropenia | Details | ||
| Filgrastim biosimilar (Qilu Pharmaceutical) | Approved | Qilu Pharmaceutical Co Ltd | 瑞白 | Mainland China | Neutropenia | Qilu Pharmaceutical Co Ltd | 1999-01-01 | Neutropenia | Details | |
| Molgramostim biosimilar (RAS Lifesciences) | Approved | Biomerieux | India | Neutropenia | Biomerieux | 2012-10-01 | Neutropenia | Details | ||
| Pegylated recombinant human granulocyte colony stimulating factor (CSPC Pharma) | Approved | CSPC Pharmaceutical Group Ltd | 津优力, Pegleukim | Mainland China | Neutropenia | Cspc Baike (Shandong) Biopharmaceutical Co Ltd | 2011-10-21 | Neutropenia | Details | |
| Filgrastim biosimilar (Dr Reddy's Laboratories) | Approved | Dr.Reddy's Laboratories Ltd | Grafeel | India | Neutropenia | Dr.Reddy's Laboratories Ltd | 2001-01-01 | Neutropenia | Details | |
| Pegfilgrastim biosimilar (Adello Biologics) | TPI-120 | Approved | Adello Biologics Llc | FYLNETRA | United States | Febrile Neutropenia | Kashiv BioSciences LLC | 2022-05-26 | Neutropenia; Febrile Neutropenia | Details |
| Pegfilgrastim biosimilar (Lupin) | Approved | Lupin Ltd | Lupifil-P | India | Neutropenia | Lupin Ltd | 2016-01-01 | Neutropenia | Details | |
| Filgrastim biosimilar (Elea) | Approved | Elea | Neutropenia | Details | ||||||
| Pegfilgrastim biosimilar (Accord Healthcare) | Approved | Accord Healthcare | Pelgraz | EU | Neutropenia | Accord Healthcare Slu | 2018-09-21 | Neutropenia | Details | |
| Pegfilgrastim biosimilar (Dr Reddy's Laboratories) | DRL_PG | Approved | Dr.Reddy's Laboratories Ltd | Peg-grafeel | India | Neutropenia | Dr.Reddy's Laboratories Ltd | 2011-01-01 | Neutropenia | Details |
| Pegfilgrastim | R-1471; SD-01; SD/01; KRN-125 | Approved | Kyowa Hakko Kirin Co Ltd, Amgen Inc | Ristempa, Neulasta, G-Lasta, Neulastim | United States | Neutropenia | Amgen Inc | 2002-01-31 | Urinary Bladder Neoplasms; Adenocarcinoma; Melanoma; Carcinoma, Non-Small-Cell Lung; Endometrial Neoplasms; Leukemia, Myeloid, Acute; Lung Neoplasms; Lymphoma; Lymphoma, Non-Hodgkin; Colorectal Neoplasms; Prostatic Neoplasms; Carcinoma, Adenosquamous; Sarcoma; Neurofibrosarcoma; Sarcoma, Ewing; Breast Neoplasms; Diabetes Mellitus, Type 1; Sarcoma, Synovial; Multiple Myeloma; Colonic Neoplasms; Small Cell Lung Carcinoma; Pancreatic Neoplasms; Carcinoma; Rectal Neoplasms; Stomach Neoplasms; Esophageal Neoplasms; Neutropenia; Ovarian Neoplasms; Solid tumours; Rhabdomyosarcoma; Head and Neck Neoplasms | Details |
| Empegfilgrastim | BCD-017 | Approved | Biocad | Extimia | Neutropenia | Details | ||||
| Pegfilgrastim biosimilar (Coherus BioSciences) | CHS-1701 | Approved | Coherus Biosciences Inc | Udenyca, UDENYCA | United States | Febrile Neutropenia | Coherus Biosciences Inc | 2018-09-21 | Neutropenia; Acute Radiation Syndrome; Febrile Neutropenia | Details |
| Filgrastim biosimilar (Institute of Medical Biology Chinese Academy of Medical Sciences) | Approved | Institute Of Medical Biology, Chinese Academy Of Medical Sciences | 吉洛因 | Mainland China | Myelodysplastic Syndromes; Leukopenia | Institute Of Medical Biology, Chinese Academy Of Medical Sciences | 2001-01-01 | Myelodysplastic Syndromes; Leukopenia | Details | |
| Pegfilgrastim biosimilar (Hospira) | HSP-130 | Approved | Pfizer Inc, Hospira | Nyvepria | United States | Febrile Neutropenia | Hospira Inc | 2020-06-10 | Neutropenia; Lymphoma, Large B-Cell, Diffuse; Lymphoma, AIDS-Related; Breast Neoplasms; Febrile Neutropenia | Details |
| Nartograstim | KW-2228; PE-2601; ND-28 | Approved | Kyowa Hakko Kirin Co Ltd | Neu-up | Japan | Neutropenia | Kyowa Hakko Kirin Co Ltd | 1994-04-01 | Neutropenia | Details |
| Pegfilgrastim biosimilar (Juta Pharma) | Approved | Juta Pharma | Grasustek | EU | Neutropenia | Juta Pharma Gmbh | 2019-04-26 | Neutropenia | Details | |
| Filgrastim biosimilar (Nanogen) | Approved | Nanogen Biopharmaceutical Co | Ficocyte | Vietnam | Neutropenia | Nanogen Biopharmaceutical Co | 2014-01-01 | Neutropenia | Details | |
| Filgrastim biosimilar (Blau Pharmaceuticals) | Approved | Blau Pharmaceuticals | Brazil | Neutropenia | Blau Pharmaceuticals | 2012-10-01 | Neutropenia | Details | ||
| Filgrastim biosimilar (Accord Healthcare) | Approved | Accord Healthcare | Accofil | EU | Neutropenia | Accord Healthcare Slu | 2014-09-17 | Neutropenia | Details | |
| Filgrastim biosimilar (PanPharmaceuticals) | Approved | Harvest Moon | India | Febrile Neutropenia | null | 2012-01-01 | Febrile Neutropenia | Details | ||
| Filgrastim biosimilar (Biosidus) | Approved | Biosidus | Neutromax, Granulostim | Neutropenia | Details | |||||
| Filgrastim biosimilar (Biocon) | Approved | Biocon Biopharmaceuticals | Nufil | India | Neutropenia | null | 2008-01-01 | Neutropenia | Details | |
| Pegfilgrastim biosimilar (Beijing SL Pharmaceutical) | Approved | Beijing Sl Pharmaceutical Co Ltd | Mainland China | Neutropenia | Beijing Sl Pharmaceutical Co Ltd | 2023-09-19 | Neutropenia | Details | ||
| Filgrastim biosimilar (Unilab) | Approved | Unilab | Neocyte | Neutropenia | Details | |||||
| Filgrastim biosimilar (GeneScience Pharmaceuticals) | Approved | Changchun GeneScience Pharmaceuticals Co Ltd | Scimax, 金磊赛强 | Mainland China | Neutropenia | Changchun GeneScience Pharmaceuticals Co Ltd | 1998-01-01 | Neutropenia | Details | |
| Pegfilgrastim biosimilar (Zydus Cadila) | Approved | Zydus Cadila | India | Neutropenia | Zydus Cadila | 2014-01-01 | Neutropenia | Details | ||
| Filgrastim biosimilar (Veropharm) | Approved | Abbott Laboratories | Mielastra | Neutropenia | Details | |||||
| Filgrastim | rhGCSF (Amgen/Kyowa Hakko Kirin); r-metHuG-CSF; REC-G-CSF; KRN-8601 | Approved | Amgen Inc, Kyowa Hakko Kirin Co Ltd | 吉粒芬, Gran, Nugraf, Neupogen, Granulokine, 惠尔血, 优保津, 特尔津, 瑞白, 吉赛欣, 津恤力 | United States | Neutropenia | Amgen Inc | 1991-02-20 | Leukemia, Myeloid, Chronic-Phase; Critical Illness; Malnutrition; Pancytopenia; Anemia, Aplastic; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Musculoskeletal Diseases; Fever; Primary Myelofibrosis; Colorectal Neoplasms; Alzheimer Disease; Breast Neoplasms; Sickle Cell Trait; Lymphoma; Ischemia; Leg Ulcer; Leukemia, Myeloid, Acute; Thrombocytopenia; Leukemia, Myelomonocytic, Juvenile; Melanoma; Crohn Disease; Respiratory Insufficiency; Cardiomyopathy, Dilated; Solid tumours; Leukemia, Myelogenous, Chronic; HIV Infections; Diabetic Foot; Neutropenia; Menopausal symptoms; Hot Flashes; Myelodysplastic Syndromes; Graft vs Host Disease; Myeloproliferative Disorders; Leukemia; Liver Cirrhosis; Anemia, Diamond-Blackfan; Pancreatic Neoplasms; Fanconi Anemia; Dyskeratosis Congenita; Stroke; Multiple Myeloma; Uveal melanoma; Shwachman-Diamond Syndrome; Neuroblastoma | Details |
| Filgrastim biosimilar (Apotex/Intas) | Approved | Apotex, Intas Biopharmaceuticals | Grastofil, SciLocyte, Neukine | India | Neutropenia | null | 2004-01-01 | Neutropenia | Details | |
| Pegfilgrastim biosimilar (Harvest Moon Pharmaceuticals/PanPharmaceuticals) | Approved | Harvest Moon | India | Neutropenia | null | 2012-01-01 | Neutropenia | Details | ||
| Filgrastim biosimilar (Pharmstandard/Masterclone/CJSC Generium) | Approved | Cjsc Generium, Pharmstandard, Masterclone | Neipomax, Neupomax | Neutropenia | null | 2008-01-01 | Neutropenia | Details | ||
| Pegfilgrastim biosimilar (Intas Biopharmaceuticals) | INTP-5 | Approved | Intas Biopharmaceuticals, Apotex | Neupeg, Lapelga, Pegasta | India | Neutropenia | null | 2007-01-01 | Hypospadias; Neutropenia; Multiple Myeloma; Lymphoma; Febrile Neutropenia | Details |
| Filgrastim biosimilar (PT Kalbe) | Approved | Kalbe Farma | India | Neutropenia | Kalbe Farma | 2012-08-01 | Neutropenia | Details | ||
| Filgrastim biosimilar (Claris Lifesciences) | Approved | Claris Lifesciences Ltd | Fegrast | India | Neutropenia | Claris Lifesciences Ltd | 2012-01-01 | Neutropenia | Details | |
| Efbemalenograstim alfa | F-627 | Approved | Evive (Shanghai) Corp Ltd | Bineuta, 亿立舒, Ryzneuta | Mainland China | Neutropenia | Evive Biopharmaceutical (Beijing) Co Ltd | 2023-05-06 | Ovarian Neoplasms; Neutropenia; Neoplasms; Breast Neoplasms; Fever; Febrile Neutropenia; Carcinoma, Non-Small-Cell Lung; Uterine Cervical Neoplasms | Details |
| Recombinant human granulocyte colony-stimulating factor (Shandong Kexing Bioproducts Co Ltd) | Approved | Shandong Kexing Bio-Products Co Ltd | 白特喜 | Mainland China | Neutropenia | Shandong Kexing Bio-Products Co Ltd | 2001-01-01 | Neutropenia | Details | |
| Filgrastim biosimilar (Apexcela) | SBS-6002; Leuco-Plus-300 | Approved | Apexcela | Neutropenia | Details | |||||
| Recombinant human granulocyte colony-stimulating Factor (Shenzhen Xinpeng Bio-tech) | Approved | Shenzhen Sinobioway Xinpeng Biomedicine Co Ltd | 瑞血新 | Mainland China | Neutropenia | Shenzhen Sinobioway Xinpeng Biomedicine Co Ltd | 1999-01-01 | Neutropenia | Details | |
| Filgrastim biosimilar (CJ Corp) | Approved | HK inno.N Corporation | Neutropenia | Details | ||||||
| Filgrastim biosimilar (Xiamen Amoytop Biotech) | Approved | Xiamen Amoytop Biotech Co Ltd | 特尔津, Topneuter | Mainland China | Neutropenia | Xiamen Amoytop Biotech Co Ltd | 1999-01-01 | Neutropenia | Details | |
| Filgrastim biosimilar (Eurofarma) | Approved | Eurofarma | Granulen | Brazil | Neutropenia | Eurofarma | 2012-01-01 | Neutropenia | Details | |
| Filgrastim biosimilar (Sedico) | Approved | Sedico | Neutropenia | Details | ||||||
| Filgrastim biosimilar (Zenotech Laboratories) | Approved | Zenotech Laboratories | Neoplasms; Neutropenia | Zenotech Laboratories | 2006-03-01 | Neutropenia; Neoplasms | Details | |||
| Filgrastim biosimilar (Lupin) | Approved | Lupin Ltd | Lupifil | India | Neutropenia | Lupin Ltd | 2016-01-01 | Neutropenia | Details | |
| Filgrastim biosimilar (Beijing SL Pharmaceutical) | Approved | Beijing Sl Pharmaceutical Co Ltd | 立生素, Li Sheng Su | Mainland China | Neutropenia | Beijing Sl Pharmaceutical Co Ltd | 1998-01-01 | Neutropenia | Details | |
| Pegfilgrastim biosimilar (Nanogen Biopharmaceutical) | Approved | Nanogen Biopharmaceutical Co | Neutropenia | Details | ||||||
| Filgrastim biosimilar (Biolotus Biotech) | Approved | Biolotus | Kymun-CSF | Brazil | Neutropenia | Biolotus | 2016-06-01 | Neutropenia | Details | |
| Filgrastim biosimilar (Fresenius Kabi Oncology) | Approved | Fresenius Kabi Ag | India | Neutropenia | Fresenius Kabi Ag | 2012-08-01 | Neutropenia | Details | ||
| Tbo-filgrastim | TKN-732; XM-02 | Approved | Teva | Tevagrastim, Biograstim, Granix | United States | Neutropenia | Sicor Biotech Uab | 2008-09-15 | Neutropenia; Multiple Myeloma; Lymphoma, Non-Hodgkin | Details |
| Filgrastim biosimilar (Laboratorios AC Farma) | Approved | Laboratorios Ac Farma | FGM-Factor | Peru | Neutropenia | Laboratorios Ac Farma | 2012-01-01 | Neutropenia | Details | |
| Filgrastim biosimilar(Pooyesh Darou Pharmaceuticals) | G-CSF | Approved | Pooyesh Darou | PD-grastim | Iran | Myelodysplastic Syndromes; Leukemia, Myeloid, Acute; Lymphoma; Neutropenia | Pooyesh Darou | 2012-09-01 | Neutropenia; Myelodysplastic Syndromes; Leukemia, Myeloid, Acute; Lymphoma | Details |
| Filgrastim biosimilar (Chandra Bhagat Pharma) | Approved | Chandra Bhagat Pharma(Cbc) | Filgen | India | Neoplasms | Chandra Bhagat Pharma(Cbc) | 2012-08-01 | Neoplasms | Details | |
| Filgrastim biosimilar (Zydus Cadila) | Approved | Zydus Cadila | India | Neutropenia | Zydus Cadila | 2014-01-01 | Neutropenia | Details | ||
| Filgrastim biosimilar (AqVida) | Approved | Aqvida Gmbh | Leucita | Russian Federation | Neutropenia | Aqvida Gmbh | 2008-05-01 | Neutropenia | Details | |
| Filgrastim biosimilar (Incepta Pharmaceuticals) | Approved | Incepta Pharmaceuticals Ltd | Neutropenia | Details | ||||||
| Pegfilgrastim biosimilar (AryaTinaGene) | Approved | Aryatinagene Biopharmaceuticals | Tinapeg | Febrile Neutropenia | Details | |||||
| Recombinant human granulocyte colony-stimulating factor (Shanghai Sunway Biotech) | Approved | Shanghai Sunway Bio Co Ltd | 赛格力, SunGran | Mainland China | Neutropenia | Shanghai Sunway Bio Co Ltd | 1998-01-01 | Neutropenia | Details | |
| Filgrastim biosimilar (Laboratorio Varifarma) | Approved | Laboratorio Varifarma | Neutrofil | Neutropenia | Details | |||||
| Recombinant human granulocyte colony-stimulating factor (Harbin Pharmaceutical Group Bioengineering) | Approved | Harbin Pharmaceutical Group Holding Co Ltd | 里亚金, Li Ya Jin | Mainland China | Neutropenia | Harbin Pharmaceutical Group Holding Co Ltd | 2000-01-01 | Neutropenia | Details | |
| Eflapegrastim | LAPS-GCSF; HNK-460; SPI-2012; HM-10460A | Approved | Hanmi Pharmaceutical Co Ltd | ROLVEDON, Rolontis, Rolvedon, ROLONTIS | South Korea | Neutropenia | Hanmi Pharmaceutical Co Ltd | 2021-03-18 | Solid tumours; Neutropenia; Neoplasms; Breast Neoplasms; Lymphoma; Febrile Neutropenia | Details |
| Recombinant Human Granulocyte Colony-stimulating Factor (Wuzhong Group) | Approved | Suzhou Pharmaceutical Factory | 洁欣, Jiexin | Mainland China | Neutropenia | Suzhou Pharmaceutical Factory | 1999-01-01 | Neutropenia | Details | |
| Filgrastim biosimilar (Chalver Laboratorios) | Approved | Chalver Laboratorios | Leucosos | Colombia | Neutropenia | Chalver Laboratorios | 2012-01-01 | Neutropenia | Details | |
| Filgrastim biosimilar (Siam Bioscience) | Approved | Siam Bioscience | Neutropenia | Details | ||||||
| Filgrastim biosimilar (Reliance Life Sciences) | Approved | Reliance Life Sciences | ReliGrast | India | Neutropenia | Reliance Life Sciences | 2008-01-01 | Neutropenia | Details | |
| Filgrastim biosimilar (Tanvex BioPharma) | TX-01 | Approved | Tanvex Biopharma | Canada | Febrile Neutropenia | Tanvex Biopharma | 2021-10-17 | Febrile Neutropenia | Details | |
| Recombinant human granulocyte stimulating factor (CSPC Pharma) | Approved | CSPC Pharmaceutical Group Ltd | 津恤力, GeneLeukim | Mainland China | Neutropenia | Cspc Baike (Shandong) Biopharmaceutical Co Ltd | 2000-01-01 | Neutropenia | Details | |
| Filgrastim biosimilar (Dong-A) | DA-3030 | Approved | Dong-A Pharmaceutical Co Ltd | Leukostim | South Korea | Neutropenia | Dong-A Pharmaceutical Co Ltd | 1999-01-01 | Drug-Related Side Effects and Adverse Reactions; Neutropenia; Diabetic Neuropathies; Breast Neoplasms | Details |
| Filgrastim biosimilar (Adello Biologics/KASHIV BIOSCIENCES) | Approved | Adello Biologics Llc | United States | Neutropenia; Febrile Neutropenia; Chemotherapy-Induced Febrile Neutropenia | Kashiv BioSciences LLC | 2022-02-25 | Chemotherapy-Induced Febrile Neutropenia; Neutropenia; Febrile Neutropenia | Details | ||
| Pegfilgrastim biosimilar (Amega Biotech) | Approved | Amega Biotech | Neutropenia | Details | ||||||
| Filgrastim biosimilar (Hangzhou Jiuyuan Gene Engineering) | Approved | Hangzhou Jiuyuan Gene Engineering Co Ltd | 吉粒芬, Jilifen | Mainland China | Neutropenia | Hangzhou Jiuyuan Gene Engineering Co Ltd | 1998-04-01 | Neutropenia | Details | |
| Filgrastim biosimilar (Probiomed) | Approved | Probiomed | Filatil | Mexico | Neutropenia | Probiomed | 2006-03-01 | Neutropenia | Details | |
| Filgrastim biosimilar (Emcure Pharmaceuticals) | Approved | Emcure Pharma | India | Neutropenia | Emcure Pharma | 2013-01-01 | Neutropenia | Details | ||
| Mecapegfilgrastim (Hengrui) | HHPG-19K | Approved | Jiangsu Hengrui Medicine Co Ltd | 艾多 | Mainland China | Neutropenia | Jiangsu Hengrui Medicine Co Ltd | 2018-05-08 | Neutropenia; Neoplasms; Breast Neoplasms; Febrile Neutropenia; Carcinoma, Non-Small-Cell Lung | Details |
| Filgrastim biosimilar (Hospira) | PLD-108 | Approved | Hospira | Nivestim, Nivestym | EU | Neutropenia; Neoplasms; Hematopoietic stem cell transplantation (HSCT) | Pfizer Europe Ma Eeig | 2010-06-07 | Hematopoietic stem cell transplantation (HSCT); Neutropenia; Neoplasms; Febrile Neutropenia | Details |
| Recombinant human granulocyte colony stimulating Factor (Beijing Four Rings Bio-Pharmaceutical) | Approved | Beijing Four Rings Bio-Pharmaceutical Co Ltd | 欣粒生, Xin Lisheng | Mainland China | Neutropenia | Beijing Four Rings Bio-Pharmaceutical Co Ltd | 2002-06-17 | Neutropenia | Details | |
| Pegfilgrastim biosimilar (Fresenius SE) | MSB-11455 | Approved | Merck Serono | Stimufend | EU | Neutropenia; Chemotherapy-Induced Febrile Neutropenia | Fresenius Kabi Deutschland Gmbh | 2022-03-28 | Chemotherapy-Induced Febrile Neutropenia; Neutropenia; Febrile Neutropenia | Details |
| Filgrastim biosimilar (Zuventus Healthcare) | Approved | Zuventus Healthcare | India | Neutropenia | Zuventus Healthcare | 2012-08-01 | Neutropenia | Details | ||
| Lenograstim biosimilar (Biosidus) | Approved | Biosidus | Lenobio, Leumostin, Lenograstim Biosidus | Neutropenia | Details | |||||
| Lipegfilgrastim | XM-22 | Approved | Teva | Lonquex | EU | Neutropenia | Teva Bv | 2013-07-25 | Rhabdomyosarcoma; Neutropenia; Multiple Myeloma; Lymphoma | Details |
| Filgrastim biosimilar (Neiss Labs) | Approved | Neiss Labs | Nisgras | India | Neutropenia | Neiss Labs | 2012-08-01 | Neutropenia | Details | |
| Filgrastim biosimilar (Fuji Pharmaceutical/Mochida/Gene Techno Science) | GBS-001; FSK-0808 | Approved | Mochida Pharmaceutical Co Ltd, Gene Techno Science, Fuji Pharmaceutical | Japan | Neutropenia | Fuji Pharmaceutical | 2012-11-21 | Neutropenia | Details | |
| Filgrastim biosimilar (Center of Molecular Immunology) | Approved | Center Of Molecular Immunology | ior-LeukoCIM | Cuba | Neutropenia | Center Of Molecular Immunology | 2016-01-14 | Neutropenia | Details | |
| Lenograstim | rG-CSF | Approved | Chugai Pharmaceutical Co Ltd | 格拉诺赛特, Neutrogin, Granocyte | Japan | Neutropenia | Chugai Pharmaceutical Co Ltd | 1991-10-04 | Neutropenia; Hodgkin Disease; Multiple Myeloma; Lymphoma, Non-Hodgkin | Details |
| Pegfilgrastim biosimilar (CinnaGen) | Approved | Cinnagen | PegaGen | Neutropenia | Details | |||||
| Pegfilgrastim biosimilar (Emcure Pharmaceuticals) | Approved | Emcure Pharma | Neutropenia | Details | ||||||
| GCPGC | MG-1107 | Approved | GC Biopharma Corp | Neulapeg | South Korea | Neutropenia | GC Biopharma Corp | 2014-01-01 | Neutropenia; Pancreatic Neoplasms | Details |
| Filgrastim biosimilar (RAS Lifesciences) | Approved | Ras Lifesciences | India | Neutropenia | Ras Lifesciences | 2012-01-01 | Neutropenia | Details | ||
| Filgrastim biosimilar (Sudershan Biotech) | Approved | Sudershan Biotech Ltd | Neutropenia | Details | ||||||
| Filgrastim biosimilar (Shandong Quangang Pharmaceutical) | Approved | Shandong Quangang Pharmaceutical Co Ltd | 泉升, Quan Sheng | Mainland China | Neutropenia | Shandong Quangang Pharmaceutical Co Ltd | 2002-04-16 | Neutropenia | Details | |
| Pegfilgrastim biosimilar (Qilu Pharmaceuticals) | PEG-rhG-CSF (Qilu Pharmaceuticals) | Approved | Qilu Pharmaceutical Co Ltd | 新瑞白 | Mainland China | Neutropenia | Qilu Pharmaceutical Co Ltd | 2015-08-08 | Neutropenia | Details |
| Filgrastim biosimilar (RPG Life Sciences) | Approved | Rpg Life Sciences Ltd | Frastim | India | Neutropenia | Rpg Life Sciences Ltd | 2012-08-01 | Neutropenia | Details | |
| Pegfilgrastim biosimilar (Sandoz) | LAEP-2006 | Approved | Clariant Produkte (Schweiz) Ag | Zioxtenzo, Ziextenzo | EU | Neutropenia | Sandoz Gmbh | 2018-11-22 | Drug-Related Side Effects and Adverse Reactions; Neutropenia; Breast Neoplasms; Febrile Neutropenia | Details |
| Telpegfilgrastim | YPEG-rhG-CSF | Approved | Xiamen Amoytop Biotech Co Ltd | 珮金 | Mainland China | Febrile Neutropenia | Xiamen Amoytop Biotech Co Ltd | 2023-06-30 | Neutropenia; Neoplasms; Breast Neoplasms; Carcinoma, Non-Small-Cell Lung; Growth hormone deficiency; Febrile Neutropenia | Details |
| Filgrastim biosimilar (Landsteiner) | Approved | Landsteiner Scientific | Biofilgran | Neutropenia | Details | |||||
| Filgrastim biosimilar (Sandoz) | EP-2006 | Approved | Sandoz | Zarxio, Zarzio | EU | Neutropenia | Hexal Ag | 2009-02-06 | Neutropenia; Breast Neoplasms; Febrile Neutropenia | Details |
| Pegfilgrastim biosimilar (Cinfa Biotech) | B-12019 | Approved | Cinfa Biotech Gmbh | Pelmeg | EU | Neutropenia | Mundipharma Corporation (Ireland) Ltd | 2018-11-20 | Neutropenia | Details |
| Filgrastim biosimilar (Pharmapark) | Approved | Pharmapark | Neutropenia | Pharmapark | 2008-01-01 | Neutropenia | Details | |||
| Filgrastim biosimilar (NCPC GeneTech Biotechnology Development) | Approved | North China Pharmaceutical Company Ltd | GeSysin | Mainland China | Neutropenia | North China Pharmaceutical Company Ltd | 1999-01-01 | Neutropenia | Details | |
| Filgrastim biosimilar (Chengdu Institute of Biological Products) | Approved | Chengdu Institute Of Biological Products Co Ltd | 保力津, Baolijin | Mainland China | Neutropenia | Chengdu Institute Of Biological Products Co Ltd | 2001-01-01 | Neutropenia | Details | |
| Pegfilgrastim biosimilar (mochida) | GBS-010; MD110 | Approved | Mochida Pharmaceutical Co Ltd | Japan | Febrile Neutropenia | Mochida Pharmaceutical Co Ltd | 2023-09-25 | Chemotherapy-Induced Febrile Neutropenia; Febrile Neutropenia | Details | |
| Recombinant Human Granulocyte/Macrophage Colony-stimulating Factor for Injection (Foshan Hanyu) | Approved | Foshan Hanyu Biopharmaceutical Co Ltd | 格宁 | Mainland China | Neutropenia | Foshan Hanyu Biopharmaceutical Co Ltd | 1998-01-01 | Neutropenia | Details | |
| Recombinant Human Granulocyte/Macrophage Colony-stimulating Factor for Injection (Beijing Beiyi Union) | Approved | Beijing Beiyi Union Pharmaceutical Co Ltd | 健白 | Mainland China | Neutropenia | Beijing Beiyi Union Pharmaceutical Co Ltd | 2002-05-08 | Neutropenia | Details | |
| Recombinant human granulocyte macrophage colony stimulating factor (Kyowa Hakko Kirin) | Approved | Kyowa Kirin Co Ltd, Ise Plant, Nipro Pharma Corp, Kyowa Kirin China Pharmaceutical Co Ltd | Details | |||||||
| Pegfilgrastim biosimilar(Shandong New Time Pharmaceutical ) | Approved | Shandong New Time Pharmaceutical Co Ltd | Details | |||||||
| Pegfilgrastim biosimilar (Biocon/Mylan) | MYL-1401H | Approved | Biocon Ltd | Fulphila | United States | Febrile Neutropenia | Mylan Gmbh | 2018-06-04 | Chemotherapy-Induced Febrile Neutropenia; Neutropenia; Breast Neoplasms; Febrile Neutropenia | Details |
| Pegfilgrastim biosimilar (Dong-A ST) | DA-3031 | Approved | Dong-A Pharmaceutical Co Ltd | Dulastin | South Korea | Neutropenia | Dong-A Pharmaceutical Co Ltd | 2014-01-01 | Solid tumours; Neutropenia; Lymphoma | Details |
| Filgrastim biosimilar (CIGB/Heber Biotec) | Approved | Center For Genetic Engineering And Biotechnology, Heber Biotec | Hebervital | Neutropenia | null | 2009-01-01 | Neutropenia | Details | ||
| Filgrastim biosimilar (Qilu Pharmaceutical) | Approved | Qilu Pharmaceutical Co Ltd | 瑞白 | Mainland China | Neutropenia | Qilu Pharmaceutical Co Ltd | 1999-01-01 | Neutropenia | Details | |
| Molgramostim biosimilar (RAS Lifesciences) | Approved | Biomerieux | India | Neutropenia | Biomerieux | 2012-10-01 | Neutropenia | Details | ||
| Pegylated recombinant human granulocyte colony stimulating factor (CSPC Pharma) | Approved | CSPC Pharmaceutical Group Ltd | 津优力, Pegleukim | Mainland China | Neutropenia | Cspc Baike (Shandong) Biopharmaceutical Co Ltd | 2011-10-21 | Neutropenia | Details | |
| Filgrastim biosimilar (Dr Reddy's Laboratories) | Approved | Dr.Reddy's Laboratories Ltd | Grafeel | India | Neutropenia | Dr.Reddy's Laboratories Ltd | 2001-01-01 | Neutropenia | Details | |
| Pegfilgrastim biosimilar (Adello Biologics) | TPI-120 | Approved | Adello Biologics Llc | FYLNETRA | United States | Febrile Neutropenia | Kashiv BioSciences LLC | 2022-05-26 | Neutropenia; Febrile Neutropenia | Details |
| Pegfilgrastim biosimilar (Lupin) | Approved | Lupin Ltd | Lupifil-P | India | Neutropenia | Lupin Ltd | 2016-01-01 | Neutropenia | Details | |
| Filgrastim biosimilar (Elea) | Approved | Elea | Neutropenia | Details | ||||||
| Pegfilgrastim biosimilar (Accord Healthcare) | Approved | Accord Healthcare | Pelgraz | EU | Neutropenia | Accord Healthcare Slu | 2018-09-21 | Neutropenia | Details | |
| Pegfilgrastim biosimilar (Dr Reddy's Laboratories) | DRL_PG | Approved | Dr.Reddy's Laboratories Ltd | Peg-grafeel | India | Neutropenia | Dr.Reddy's Laboratories Ltd | 2011-01-01 | Neutropenia | Details |
| English Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| Pegfilgrastim biosimilar (Reliance Life Sciences) | R-TPR-029 | Phase 3 Clinical | Reliance Life Sciences | Neutropenia | Details |
| Filgrastim biosimilar (Bio-Ker) | BK-0023 | Phase 3 Clinical | Bio-Ker Srl | Neutropenia | Details |
| Filgrastim biosimilar (Virchow Group) | Neutrogen | Phase 3 Clinical | Virchow Group | Neutropenia | Details |
| Filgrastim biosimilar(Blau Farmaceutica) | Phase 3 Clinical | Blau Farmaceutica Sa | Breast Neoplasms | Details | |
| G-CSF(GPCR Therapeutics) | G-CSF(GPCR Therapeutics) | Phase 3 Clinical | GPCR Therapeutics USA Inc | Multiple Myeloma; Neuroblastoma; Ganglioneuroblastoma | Details |
| Pegfilgrastim ANF | PEG-G-CSF-ANF | Phase 2 Clinical | Prolong Pharmaceuticals Llc | Neutropenia | Details |
| GM-CSF / IL3 fusion protein | Phase 2 Clinical | Shanghai Zhao'An Medical Technology Co Ltd, Beijing Igus Medical Technology Development Co Ltd | Neuroblastoma | Details | |
| Recombinant Human Granulocyte Colony-Stimulating Factor(Children's Hospital of Soochow University) | Phase 2 Clinical | Children's Hospital of Soochow University | Leukemia, Myeloid, Acute | Details | |
| JZB-27 | JZB-27 | Phase 2 Clinical | Shanghai Jingze Biotechnology Co Ltd, Hunan Jingfeng Pharmaceutical Co Ltd | Neutropenia | Details |
| Albumin-granulocyte colony stimulating factor (Beijing BioFortune/Tianjin SinoBiotech) | SFR-9314; rHSA/GCSF | Phase 2 Clinical | Beijing Meifuyuan Biomedical Technology Co Ltd, Tianjin Puying Biotechnology Co Ltd | Chemotherapy-Induced Febrile Neutropenia; Neutropenia; Agranulocytosis | Details |
| Anumigilimab | CSL-324 | Phase 1 Clinical | Csl Ltd, Murigen | Coronavirus Disease 2019 (COVID-19); Psoriasis; Hidradenitis Suppurativa | Details |
| Filgrastim biosimilar (Biogenomics) | Phase 1 Clinical | Biogenomics | Neutropenia | Details | |
| PEGylated G-CSF | BBT-015 | Phase 1 Clinical | Bolder Biotechnology | Neutropenia; Acute Radiation Syndrome | Details |
| Pegfilgrastim biosimilar (Bio-Ker) | Phase 1 Clinical | Bio-Ker Srl | Neutropenia | Details | |
| Pegfilgrastim biosimilar (Jiangsu Aosaikang Pharmaceutical) | Phase 1 Clinical | Jiangsu Aosaikang Pharmaceutical Co Ltd | Solid tumours | Details | |
| Granulocyte colony-stimulating factor(Kura Oncology Inc) | Phase 1 Clinical | Kura Oncology Inc | Leukemia; Leukemia, Myeloid; Hematologic Neoplasms; Neoplasms; Leukemia, Myeloid, Acute | Details | |
| Recombinant human granulocyte-stimulating factor(Zhongnan Hospital) | Phase 1 Clinical | Zhongnan Hospital Of Wuhan University | Hematologic Neoplasms | Details | |
| Polyethylene glycolized recombinant human granulocyte-stimulating factor(Zhongnan Hospital) | Phase 1 Clinical | Zhongnan Hospital Of Wuhan University | Hematologic Neoplasms | Details | |
| Filgrastim biosimilar (MenoGenix) | MNGX-100 | Phase 1 Clinical | Menogenix Inc | Menopausal symptoms | Details |
| Pegfilgrastim biosimilar (Megalabs) | Phase 1 Clinical | Megalabs | Neutropenia | Details | |
| QL-0605 | PEG-rhG-CSF | Phase 1 Clinical | Qilu Pharmaceutical Co Ltd | Details | |
| Pegfilgrastim biosimilar (Affiliated Hospital of North Sichuan Medical College) | Clinical | Affiliated Hospital Of North Sichuan Medical College | Neutropenia | Details | |
| BSC-0826 | BSC-0826 | Clinical | Biosimilar Solutions Inc | Neutropenia; Radiation Injuries | Details |
| Pegfilgrastim biosimilar (Reliance Life Sciences) | R-TPR-029 | Phase 3 Clinical | Reliance Life Sciences | Neutropenia | Details |
| Filgrastim biosimilar (Bio-Ker) | BK-0023 | Phase 3 Clinical | Bio-Ker Srl | Neutropenia | Details |
| Filgrastim biosimilar (Virchow Group) | Neutrogen | Phase 3 Clinical | Virchow Group | Neutropenia | Details |
| Filgrastim biosimilar(Blau Farmaceutica) | Phase 3 Clinical | Blau Farmaceutica Sa | Breast Neoplasms | Details | |
| G-CSF(GPCR Therapeutics) | G-CSF(GPCR Therapeutics) | Phase 3 Clinical | GPCR Therapeutics USA Inc | Multiple Myeloma; Neuroblastoma; Ganglioneuroblastoma | Details |
| Pegfilgrastim ANF | PEG-G-CSF-ANF | Phase 2 Clinical | Prolong Pharmaceuticals Llc | Neutropenia | Details |
| GM-CSF / IL3 fusion protein | Phase 2 Clinical | Shanghai Zhao'An Medical Technology Co Ltd, Beijing Igus Medical Technology Development Co Ltd | Neuroblastoma | Details | |
| Recombinant Human Granulocyte Colony-Stimulating Factor(Children's Hospital of Soochow University) | Phase 2 Clinical | Children's Hospital of Soochow University | Leukemia, Myeloid, Acute | Details | |
| JZB-27 | JZB-27 | Phase 2 Clinical | Shanghai Jingze Biotechnology Co Ltd, Hunan Jingfeng Pharmaceutical Co Ltd | Neutropenia | Details |
| Albumin-granulocyte colony stimulating factor (Beijing BioFortune/Tianjin SinoBiotech) | SFR-9314; rHSA/GCSF | Phase 2 Clinical | Beijing Meifuyuan Biomedical Technology Co Ltd, Tianjin Puying Biotechnology Co Ltd | Chemotherapy-Induced Febrile Neutropenia; Neutropenia; Agranulocytosis | Details |
| Anumigilimab | CSL-324 | Phase 1 Clinical | Csl Ltd, Murigen | Coronavirus Disease 2019 (COVID-19); Psoriasis; Hidradenitis Suppurativa | Details |
| Filgrastim biosimilar (Biogenomics) | Phase 1 Clinical | Biogenomics | Neutropenia | Details | |
| PEGylated G-CSF | BBT-015 | Phase 1 Clinical | Bolder Biotechnology | Neutropenia; Acute Radiation Syndrome | Details |
| Pegfilgrastim biosimilar (Bio-Ker) | Phase 1 Clinical | Bio-Ker Srl | Neutropenia | Details | |
| Pegfilgrastim biosimilar (Jiangsu Aosaikang Pharmaceutical) | Phase 1 Clinical | Jiangsu Aosaikang Pharmaceutical Co Ltd | Solid tumours | Details | |
| Granulocyte colony-stimulating factor(Kura Oncology Inc) | Phase 1 Clinical | Kura Oncology Inc | Leukemia; Leukemia, Myeloid; Hematologic Neoplasms; Neoplasms; Leukemia, Myeloid, Acute | Details | |
| Recombinant human granulocyte-stimulating factor(Zhongnan Hospital) | Phase 1 Clinical | Zhongnan Hospital Of Wuhan University | Hematologic Neoplasms | Details | |
| Polyethylene glycolized recombinant human granulocyte-stimulating factor(Zhongnan Hospital) | Phase 1 Clinical | Zhongnan Hospital Of Wuhan University | Hematologic Neoplasms | Details | |
| Filgrastim biosimilar (MenoGenix) | MNGX-100 | Phase 1 Clinical | Menogenix Inc | Menopausal symptoms | Details |
| Pegfilgrastim biosimilar (Megalabs) | Phase 1 Clinical | Megalabs | Neutropenia | Details | |
| QL-0605 | PEG-rhG-CSF | Phase 1 Clinical | Qilu Pharmaceutical Co Ltd | Details | |
| Pegfilgrastim biosimilar (Affiliated Hospital of North Sichuan Medical College) | Clinical | Affiliated Hospital Of North Sichuan Medical College | Neutropenia | Details | |
| BSC-0826 | BSC-0826 | Clinical | Biosimilar Solutions Inc | Neutropenia; Radiation Injuries | Details |
This web search service is supported by Google Inc.





