 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!
 Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!  Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!
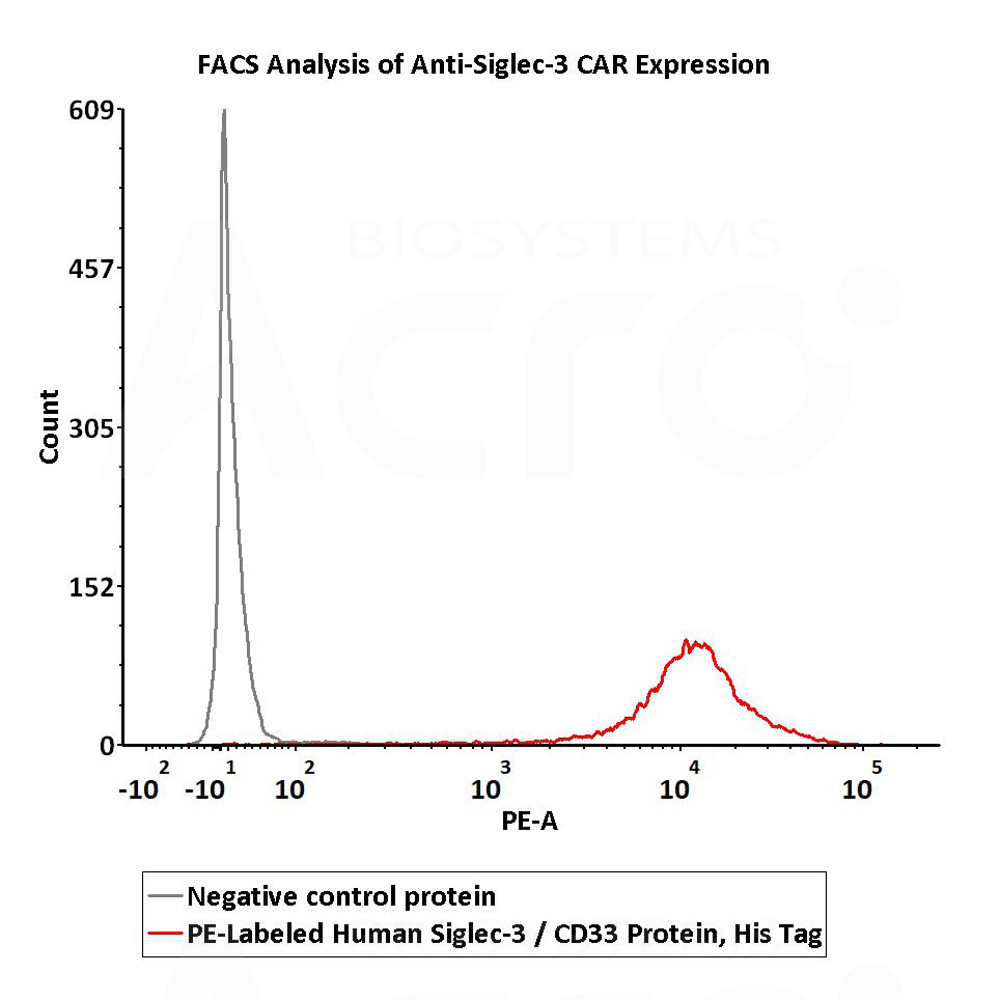
5e5 of anti-Siglec-3 CAR-293 cells were stained with 100 μL of 1:50 dilution (2 μL stock solution in 100 μL FACS buffer) of PE-Labeled Human Siglec-3, His Tag (Cat. No. CD3-HP2E3) and negative control protein respectively. PE signal was used to evaluate the binding activity (QC tested).

Loaded Siglec-3 MAb (Mouse IgG1) on AMC Biosensor, can bind Human Siglec-3 Protein, Llama IgG2b Fc Tag (Cat. No. CD3-H5259) with an affinity constant of 0.365 nM as determined in BLI assay (ForteBio Octet Red96e) (Routinely tested).
| Name | Research Code | Research Phase | Company | First Brand Name | First Approved Country | First Indication | First Approved Company | First Approved Date | Indications | Clinical Trials |
|---|---|---|---|---|---|---|---|---|---|---|
| Gemtuzumab ozogamicin | WAY-CMA-676; CL-555201; CMA-676; CDP-771; hP67.6-calicheamicin | Approved | Pfizer Inc, Ucb Sa | Mylotarg | Japan | Leukemia, Myeloid, Acute | Pfizer Inc | 2008-01-18 | Leukemia; Myelodysplastic Syndromes; Leukemia, Myeloid, Acute; Leukemia, Myelomonocytic, Juvenile; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
| Gemtuzumab ozogamicin | WAY-CMA-676; CL-555201; CMA-676; CDP-771; hP67.6-calicheamicin | Approved | Pfizer Inc, Ucb Sa | Mylotarg | Japan | Leukemia, Myeloid, Acute | Pfizer Inc | 2008-01-18 | Leukemia; Myelodysplastic Syndromes; Leukemia, Myeloid, Acute; Leukemia, Myelomonocytic, Juvenile; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| BI-836858 | BI-836858 | Phase 2 Clinical | C.H. Boehringer Sohn Ag & Co. Kg | Myelodysplastic Syndromes; Leukemia, Myeloid, Acute | Details |
| CART-33 (Chinese PLA General Hospital) | Phase 2 Clinical | Pla General Hospital | Leukemia, Myeloid; Leukemia, Myeloid, Acute | Details | |
| Lintuzumab Ac-225 (Actinium Pharmaceuticals) | 225Ac-HuM-195; HuM195-Ac-225; AC225-MOAB-M195 | Phase 2 Clinical | Pdl Biopharma Inc | Multiple Myeloma; Leukemia, Myeloid, Acute | Details |
| VCAR-33 (Vor Biopharma) | VCAR-33; VCAR33 | Phase 2 Clinical | Vor Biopharma Inc | Leukemia, Myeloid, Acute | Details |
| MP0533 | MP0533 | Phase 2 Clinical | Molecular Partners Ag | Leukemia, Myeloid, Acute | Details |
| CD33 CAR T Cells(Beijing Boren Hospital) | Phase 2 Clinical | Beijing Gao Boren Hospital Co Ltd | Leukemia; Neoplasms; Leukemia, Myeloid, Acute | Details | |
| Emerfetamab | AMG-673; AMG673 | Phase 1 Clinical | Amgen Inc | Leukemia, Myeloid, Acute | Details |
| ICG-136 | ICG-136; 123b-33bcCAR | Phase 1 Clinical | Icell Gene Therapeutics (Int'L) Ltd | Leukemia, Myeloid, Acute | Details |
| Vixtimotamab | T-564; AMV-564 | Phase 1 Clinical | Amphivena Therapeutics Inc | Solid tumours; Myelodysplastic Syndromes | Details |
| AL-003 | AL-003 | Phase 1 Clinical | Abbvie Inc, Alector Inc | Alzheimer Disease | Details |
| PRGN-3006 | PRGN-3006 | Phase 1 Clinical | Precigen Inc | Myelodysplastic Syndromes; Leukemia, Myeloid, Acute | Details |
| IM73 chimeric antigen receptor T cell therapy | IM-73-CAR-T | Phase 1 Clinical | Beijing Immunochina Medical Science & Technology Co Ltd | Leukemia, Myeloid, Acute | Details |
| ORM-6151 | ORM-6151 | Phase 1 Clinical | Orum Therapeutics Inc | Myelodysplastic Syndromes; Leukemia, Myeloid, Acute | Details |
| SENTI-202 | SENTI-202 | Phase 1 Clinical | Senti Biosciences Inc | Hematologic Neoplasms; Myelodysplastic Syndromes; Leukemia, Myeloid, Acute | Details |
| JNJ-67571244 | JNJ-67571244; JNJ-67371244; JNJ-1244 | Phase 1 Clinical | Johnson & Johnson | Myelodysplastic Syndromes; Leukemia, Myeloid, Acute | Details |
| CD33-NKE | CD33-NKE; CD33 NKE | Phase 1 Clinical | Bristol-Myers Squibb Company | Leukemia, Myeloid, Acute | Details |
| Optimised CD33 (FL-33) CAR T Therapy(Beijing GoBroad Hospital) | Phase 1 Clinical | Beijing GoBroad Hospital | Leukemia, Myeloid, Acute | Details | |
| Universal CAR-T Cells therapy(Shenzhen Geno-Immune Medical Institute) | Phase 1 Clinical | Shenzhen Geno-Immune Medical Institute | Leukemia, Myeloid, Acute | Details | |
| CD33/CLL1 dual CAR-NK cell therapy(Institute Of Hematology & Blood Diseases Hospital) | Phase 1 Clinical | Institute Of Hematology & Blood Diseases Hospital, Hangzhou Qihan Biotechnology Co Ltd | Neoplasm, Residual; Leukemia, Myeloid, Acute | Details | |
| CD33 CAR-NK cell therapy(Institute Of Hematology & Blood Diseases Hospital) | Phase 1 Clinical | Institute Of Hematology & Blood Diseases Hospital, Hangzhou Qihan Biotechnology Co Ltd | Neoplasm, Residual; Leukemia, Myeloid, Acute | Details | |
| IMGN-779 | IMGN-779 | Phase 1 Clinical | Immunogen Inc | Leukemia, Myeloid, Acute | Details |
| Eluvixtamab | MT-114; AMG-330 | Phase 1 Clinical | Amgen Inc | Myelodysplastic Syndromes; Leukemia, Myeloid, Acute | Details |
| CLL-1/CD33 Targeted LCAR-AMDR Cells(Nanjing Legend Biotechnology) | Phase 1 Clinical | Leukemia, Myeloid, Acute | Details | ||
| IBR-733 | IBR-733; IBR733 | Phase 1 Clinical | Imbioray (Hangzhou) Biomedicine Co Ltd | Leukemia, Myeloid, Acute | Details |
| CD33 CAR-T Therapy (Zhejiang University) | Phase 1 Clinical | Zhejiang University | Leukemia, Myeloid, Acute | Details | |
| CLL1+CD33 CAR-T(Zhejiang University) | Phase 1 Clinical | Zhejiang University | Leukemia, Myeloid, Acute | Details | |
| anti-CD33 CAR T cells therapy(iCell Gene Therapeutics) | Phase 1 Clinical | Icell Gene Therapeutics (Int'L) Ltd | Hematologic Neoplasms; Leukemia, Myeloid, Acute | Details | |
| Anti-CD33/CLL1 CAR-NK Cell Therapy | Phase 1 Clinical | Imbioray (Hangzhou) Biomedicine Co Ltd | Leukemia, Myeloid, Acute | Details | |
| Lintuzumab Ac-225/Venetoclax | Phase 1 Clinical | Actinium Pharmaceuticals Inc | Leukemia, Promyelocytic, Acute | Details | |
| DXC-007(Hangzhou Dac Biotech Company) | DXC-007 | Phase 1 Clinical | Hangzhou Dac Biotech Company Ltd | Leukemia; Leukemia, Myeloid, Acute | Details |
| Lintuzumab-gelonin conjugate | Phase 1 Clinical | The University Of Texas MD Anderson Cancer Center | Leukemia, Myelogenous, Chronic; Myelodysplastic Syndromes; Leukemia, Myeloid, Acute | Details | |
| Anti-CD33/CLL-1 CAR-T (Legend) | Phase 1 Clinical | Legend Biotech Corp | Leukemia, Promyelocytic, Acute | Details | |
| Anti-CD33/anti-FcgammaRI bispecific antibody (UCSD) | Phase 1 Clinical | University Of California San Diego | Leukemia, Myeloid, Acute | Details | |
| Anti-CD33 CAR T cell therapy (Wuhan Bio-Raid) | Clinical | Wuhan BioRaid Biotechnology Co Ltd | Leukemia, Myeloid, Acute | Details | |
| BI-836858 | BI-836858 | Phase 2 Clinical | C.H. Boehringer Sohn Ag & Co. Kg | Myelodysplastic Syndromes; Leukemia, Myeloid, Acute | Details |
| CART-33 (Chinese PLA General Hospital) | Phase 2 Clinical | Pla General Hospital | Leukemia, Myeloid; Leukemia, Myeloid, Acute | Details | |
| Lintuzumab Ac-225 (Actinium Pharmaceuticals) | 225Ac-HuM-195; HuM195-Ac-225; AC225-MOAB-M195 | Phase 2 Clinical | Pdl Biopharma Inc | Multiple Myeloma; Leukemia, Myeloid, Acute | Details |
| VCAR-33 (Vor Biopharma) | VCAR-33; VCAR33 | Phase 2 Clinical | Vor Biopharma Inc | Leukemia, Myeloid, Acute | Details |
| MP0533 | MP0533 | Phase 2 Clinical | Molecular Partners Ag | Leukemia, Myeloid, Acute | Details |
| CD33 CAR T Cells(Beijing Boren Hospital) | Phase 2 Clinical | Beijing Gao Boren Hospital Co Ltd | Leukemia; Neoplasms; Leukemia, Myeloid, Acute | Details | |
| Emerfetamab | AMG-673; AMG673 | Phase 1 Clinical | Amgen Inc | Leukemia, Myeloid, Acute | Details |
| ICG-136 | ICG-136; 123b-33bcCAR | Phase 1 Clinical | Icell Gene Therapeutics (Int'L) Ltd | Leukemia, Myeloid, Acute | Details |
| Vixtimotamab | T-564; AMV-564 | Phase 1 Clinical | Amphivena Therapeutics Inc | Solid tumours; Myelodysplastic Syndromes | Details |
| AL-003 | AL-003 | Phase 1 Clinical | Abbvie Inc, Alector Inc | Alzheimer Disease | Details |
| PRGN-3006 | PRGN-3006 | Phase 1 Clinical | Precigen Inc | Myelodysplastic Syndromes; Leukemia, Myeloid, Acute | Details |
| IM73 chimeric antigen receptor T cell therapy | IM-73-CAR-T | Phase 1 Clinical | Beijing Immunochina Medical Science & Technology Co Ltd | Leukemia, Myeloid, Acute | Details |
| ORM-6151 | ORM-6151 | Phase 1 Clinical | Orum Therapeutics Inc | Myelodysplastic Syndromes; Leukemia, Myeloid, Acute | Details |
| SENTI-202 | SENTI-202 | Phase 1 Clinical | Senti Biosciences Inc | Hematologic Neoplasms; Myelodysplastic Syndromes; Leukemia, Myeloid, Acute | Details |
| JNJ-67571244 | JNJ-67571244; JNJ-67371244; JNJ-1244 | Phase 1 Clinical | Johnson & Johnson | Myelodysplastic Syndromes; Leukemia, Myeloid, Acute | Details |
| CD33-NKE | CD33-NKE; CD33 NKE | Phase 1 Clinical | Bristol-Myers Squibb Company | Leukemia, Myeloid, Acute | Details |
| Optimised CD33 (FL-33) CAR T Therapy(Beijing GoBroad Hospital) | Phase 1 Clinical | Beijing GoBroad Hospital | Leukemia, Myeloid, Acute | Details | |
| Universal CAR-T Cells therapy(Shenzhen Geno-Immune Medical Institute) | Phase 1 Clinical | Shenzhen Geno-Immune Medical Institute | Leukemia, Myeloid, Acute | Details | |
| CD33/CLL1 dual CAR-NK cell therapy(Institute Of Hematology & Blood Diseases Hospital) | Phase 1 Clinical | Institute Of Hematology & Blood Diseases Hospital, Hangzhou Qihan Biotechnology Co Ltd | Neoplasm, Residual; Leukemia, Myeloid, Acute | Details | |
| CD33 CAR-NK cell therapy(Institute Of Hematology & Blood Diseases Hospital) | Phase 1 Clinical | Institute Of Hematology & Blood Diseases Hospital, Hangzhou Qihan Biotechnology Co Ltd | Neoplasm, Residual; Leukemia, Myeloid, Acute | Details | |
| IMGN-779 | IMGN-779 | Phase 1 Clinical | Immunogen Inc | Leukemia, Myeloid, Acute | Details |
| Eluvixtamab | MT-114; AMG-330 | Phase 1 Clinical | Amgen Inc | Myelodysplastic Syndromes; Leukemia, Myeloid, Acute | Details |
| CLL-1/CD33 Targeted LCAR-AMDR Cells(Nanjing Legend Biotechnology) | Phase 1 Clinical | Leukemia, Myeloid, Acute | Details | ||
| IBR-733 | IBR-733; IBR733 | Phase 1 Clinical | Imbioray (Hangzhou) Biomedicine Co Ltd | Leukemia, Myeloid, Acute | Details |
| CD33 CAR-T Therapy (Zhejiang University) | Phase 1 Clinical | Zhejiang University | Leukemia, Myeloid, Acute | Details | |
| CLL1+CD33 CAR-T(Zhejiang University) | Phase 1 Clinical | Zhejiang University | Leukemia, Myeloid, Acute | Details | |
| anti-CD33 CAR T cells therapy(iCell Gene Therapeutics) | Phase 1 Clinical | Icell Gene Therapeutics (Int'L) Ltd | Hematologic Neoplasms; Leukemia, Myeloid, Acute | Details | |
| Anti-CD33/CLL1 CAR-NK Cell Therapy | Phase 1 Clinical | Imbioray (Hangzhou) Biomedicine Co Ltd | Leukemia, Myeloid, Acute | Details | |
| Lintuzumab Ac-225/Venetoclax | Phase 1 Clinical | Actinium Pharmaceuticals Inc | Leukemia, Promyelocytic, Acute | Details | |
| DXC-007(Hangzhou Dac Biotech Company) | DXC-007 | Phase 1 Clinical | Hangzhou Dac Biotech Company Ltd | Leukemia; Leukemia, Myeloid, Acute | Details |
| Lintuzumab-gelonin conjugate | Phase 1 Clinical | The University Of Texas MD Anderson Cancer Center | Leukemia, Myelogenous, Chronic; Myelodysplastic Syndromes; Leukemia, Myeloid, Acute | Details | |
| Anti-CD33/CLL-1 CAR-T (Legend) | Phase 1 Clinical | Legend Biotech Corp | Leukemia, Promyelocytic, Acute | Details | |
| Anti-CD33/anti-FcgammaRI bispecific antibody (UCSD) | Phase 1 Clinical | University Of California San Diego | Leukemia, Myeloid, Acute | Details | |
| Anti-CD33 CAR T cell therapy (Wuhan Bio-Raid) | Clinical | Wuhan BioRaid Biotechnology Co Ltd | Leukemia, Myeloid, Acute | Details |
This web search service is supported by Google Inc.
