 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!
 Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!  Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!
> Insights > Rebalancing the Immune System: How Cell Therapies are Revolutionizing the Treatment of Autoimmune Diseases Autoimmune diseases are chronic, debilitating conditions characterized by the immune system mistakenly attacking the body's own healthy tissues, leading to inflammation and organ damage. These diseases, which include rheumatoid arthritis, lupus, multiple sclerosis, and inflammatory bowel disease, affect millions of people globally. They result from a dysfunction in the immune system's ability to distinguish self from non-self, triggering an aberrant immune response against the body's own cells and tissues.
Recently, chimeric antigen receptor (CAR) cell therapy has emerged as a promising solution for treating autoimmune diseases. CAR cell therapy involves engineering immune cells, such as T cells or natural killer (NK) cells, to express specialized receptors that can target and eliminate specific cells driving the autoimmune process. By precisely targeting and depleting the pathogenic immune cells responsible for the autoimmune attack, CAR cell therapies offer the potential to rebalance the dysregulated immune system and halt disease progression.
CAR-T cell therapy is a leading approach in the field of autoimmune disease treatment. This technology involves genetically modifying a patient's own T cells to express chimeric antigen receptors (CARs) that recognize specific proteins, such as CD19, expressed on the surface of B cells. By targeting and eliminating the autoreactive B cells that produce pathogenic antibodies, CAR-T cell therapy can effectively reduce the autoimmune response.
Initial studies have shown promising results in the treatment of systemic lupus erythematosus (SLE), a complex autoimmune condition affecting multiple organs. CAR-T cells targeting CD19 have demonstrated the ability to deplete circulating B cells, leading to significant clinical improvements in SLE patients. Researchers have also developed a specialized variant called chimeric autoantibody receptor (CAAR) T cells, which are designed to specifically target and eliminate B cells that produce disease-causing autoantibodies, further enhancing the precision of this approach.
Another innovative strategy involves the use of CAR-modified T regulatory cells (Tregs) to suppress the activity of autoreactive cells, promoting immune tolerance and reducing inflammation.
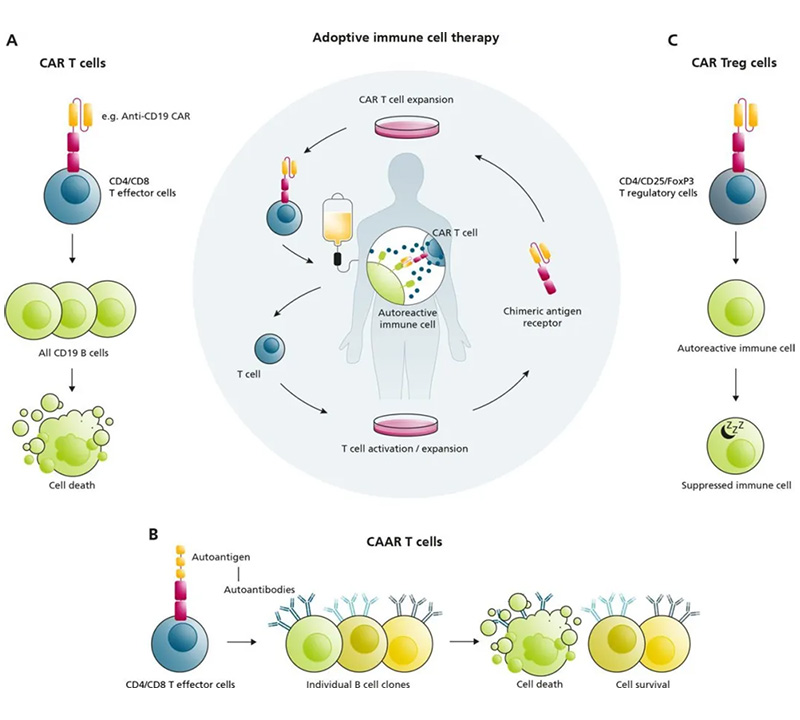
Figure 1. Adoptive Immune Cell Therapy Using CAR T Cells for Autoimmune Diseases.
CAR-NK cell therapy is an emerging approach that harnesses the natural cytotoxic abilities of natural killer (NK) cells. Like CAR-T cells, CAR-NK cells are genetically engineered to express chimeric antigen receptors that enable them to target and eliminate specific cell types involved in autoimmune diseases. CAR-NK cell therapy offers several potential advantages over CAR-T cells, including a potentially better safety profile due to a lower risk of cytokine release syndrome and graft-versus-host disease.
Recent studies have demonstrated the potential of CAR-NK cells in treating autoimmune conditions such as SLE. For instance, CAR-NK92 cells have been shown to effectively target and kill follicular helper T cells, which play a critical role in the pathogenesis of SLE. By reducing the proliferation of these memory B cell-supporting cells and decreasing immunoglobulin secretion, CAR-NK cell therapy can significantly alleviate disease symptoms.
Several biopharmaceutical companies are at the forefront of advancing CAR-T and CAR-NK cell therapies for autoimmune diseases:
- iCell Gene Therapeutics presented promising results from a Phase I clinical trial targeting systemic lupus erythematosus (SLE). The study showed that 11 out of 12 patients treated with CAR-T cells had all autoantibodies eliminated and maintained disease remission without medication for up to 4.5 years.
- Kyverna Therapeutics released initial clinical trial results demonstrating the potential of CAR-T cell therapy in clearing pathogenic B cells causing multiple sclerosis. The company is pioneering CAR T-cell therapies for autoimmune diseases, aiming to achieve sustained treatment-free remission by resetting the immune system.
- Takeda Pharmaceutical announced a shift in focus for its allogeneic CAR-NK cell therapy, TAK-007, from hematologic cancers to autoimmune diseases. Clinical trials for lupus nephritis are expected to begin next year.
- Novartis is exploring ways to improve the benefit-risk profile and conditioning process of CAR-T therapies for autoimmune diseases, with the goal of enabling earlier treatment intervention in patients.
- Bristol Myers Squibb is also investing in CAR-T research for autoimmune diseases, recognizing the significant potential for patient benefit in this area.
- Gracell Biotechnologies, recently acquired by AstraZeneca, is developing CAR-T therapies targeting CD19 for autoimmune diseases.
Mechanism of Action and Cytokine Secretion The effectiveness of CAR cell therapies is partly attributed to their ability to induce the secretion of cytokines, which enhance the immune response against target cells. CAR-T cells, for instance, can mediate target cell killing through pathways involving perforin and granzyme, as well as the release of pro-inflammatory cytokines like IFN-γ, IL-2, and TNF-α. The Fas/FasL pathway is another mechanism by which CAR-T cells can induce apoptosis in target cells.
Monitoring the levels of these cytokines is crucial for evaluating the efficacy and safety of CAR cell therapies, as elevated cytokine levels can contribute to the development of adverse events, such as cytokine release syndrome.
CAR-T and CAR-NK cell therapies represent a significant advancement in the treatment of autoimmune diseases. By specifically targeting and eliminating the pathogenic immune cells driving the autoimmune response, these therapies offer a more precise and potentially safer alternative to traditional immunosuppressant medications. Ongoing research and clinical trials continue to refine these approaches, promising new pathways for the effective management of autoimmune conditions.
ACROBiosystems is providing you with a series of recombinant cytokines and their receptors, including Interleukins, Growth Factors, TNFs, Chemokines, CSFs, IFNs, and Complement Components with high purity, high bioactivity, and high batch-to-batch consistency to accelerate your autoimmune diseases drug development programs.
We have launched ClinMax™ ready-to-use ELISA kits with rigorously quality control, ensuring the precision, stability, and consistency of the analysis results, to better meet your experimental needs.
1. Zhong Y, Liu J. Emerging roles of CAR-NK cell therapies in tumor immunotherapy: current status and future directions. Cell Death Discov. 2024;10(1):318. Published 2024 Jul 10. doi:10.1038/s41420-024-02077-1
2. Liu Y, Dong M, Chu Y, et al. Dawn of CAR-T cell therapy in autoimmune diseases. Chin Med J (Engl). 2024;137(10):1140-1150. doi:10.1097/CM9.0000000000003111
3. Kilgour, Marisa K et al. “Advancements in CAR-NK therapy: lessons to be learned from CAR-T therapy.” Frontiers in immunology vol. 14 1166038. 2 May. 2023, doi:10.3389/fimmu.2023.1166038
4. Műzes, Györgyi, and Ferenc Sipos. “CAR-Based Therapy for Autoimmune Diseases: A Novel Powerful Option.” Cells vol. 12,11 1534. 2 Jun. 2023, doi:10.3390/cells12111534
5. Reighard SD, Cranert SA, Rangel KM, et al. Therapeutic Targeting of Follicular T Cells with Chimeric Antigen Receptor-Expressing Natural Killer Cells [published correction appears in Cell Rep Med. 2020 Aug 25;1(5):100080. doi: 10.1016/j.xcrm.2020.100080]. Cell Rep Med. 2020;1(1):100003. doi:10.1016/j.xcrm.2020.100003
This web search service is supported by Google Inc.
