 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!
 Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!  Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!
| 제품번호 | 종 | 제품 설명 | 구조 | 순도 | 특징 |
|---|---|---|---|---|---|
| TY3-H5251 | Human | Human TYRO3 / Dtk Protein, Fc Tag |  |
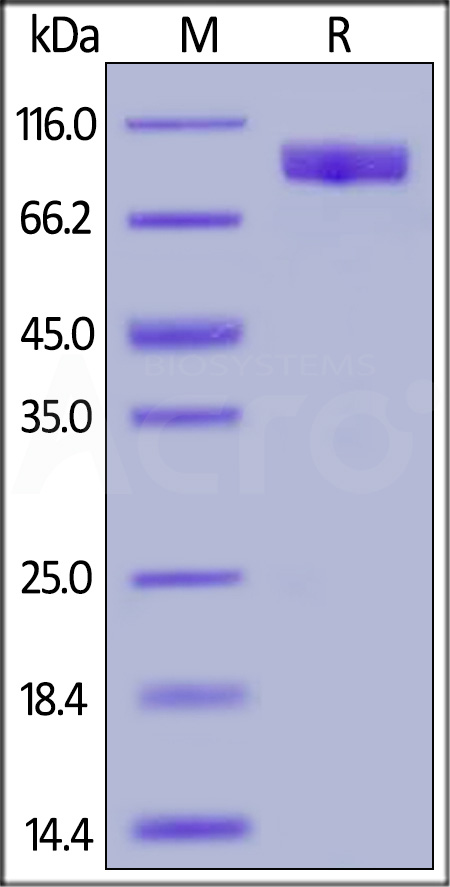
|
| Name | Research Code | Research Phase | Company | First Brand Name | First Approved Country | First Indication | First Approved Company | First Approved Date | Indications | Clinical Trials |
|---|---|---|---|---|---|---|---|---|---|---|
| Cabozantinib S-malate | XL-184; BMS-907351 | Approved | Exelixis Inc | Cometriq, Cabometyx | United States | Carcinoma, Neuroendocrine; Thyroid Neoplasms | Exelixis Inc | 2012-11-29 | Urethral Neoplasms; Leukemia, Myeloid, Acute; Thyroid Neoplasms; Gliosarcoma; Colorectal Neoplasms; Astrocytoma; Hepatic Insufficiency; Peritoneal Neoplasms; Bile Duct Neoplasms; Sarcoma, Clear Cell; Uterine Neoplasms; Adenocarcinoma, Clear Cell; Sarcoma, Ewing; Carcinoma, Adenosquamous; Breast Neoplasms; Neurofibroma, Plexiform; Osteosarcoma; Prostatic Neoplasms; Sarcoma; Medullary thyroid cancer (MTC); Carcinoma, Hepatocellular; Melanoma; Paraganglioma; Meningioma; Neoplasms, Germ Cell and Embryonal; Neoplasm Metastasis; Carcinoma, Non-Small-Cell Lung; Adenocarcinoma; Carcinoma, Endometrioid; Thyroid Cancer, Papillary; Brain Neoplasms; Carcinoma, Neuroendocrine; Sarcoma, Alveolar Soft Part; Lymphoma; Fallopian Tube Neoplasms; Brain metastases; Glioma; Endometrial Neoplasms; Carcinoma, Squamous Cell; Carcinoid Tumor; Glioblastoma; Skin Neoplasms; Neoplasms; Carcinoma, Papillary; Hepatoblastoma; Pheochromocytoma; Pain; Rejection of liver transplantation; Carcinoma, Renal Cell; Pancreatic neuroendocrine tumors | Details |
| Cabozantinib S-malate | XL-184; BMS-907351 | Approved | Exelixis Inc | Cometriq, Cabometyx | United States | Carcinoma, Neuroendocrine; Thyroid Neoplasms | Exelixis Inc | 2012-11-29 | Urethral Neoplasms; Leukemia, Myeloid, Acute; Thyroid Neoplasms; Gliosarcoma; Colorectal Neoplasms; Astrocytoma; Hepatic Insufficiency; Peritoneal Neoplasms; Bile Duct Neoplasms; Sarcoma, Clear Cell; Uterine Neoplasms; Adenocarcinoma, Clear Cell; Sarcoma, Ewing; Carcinoma, Adenosquamous; Breast Neoplasms; Neurofibroma, Plexiform; Osteosarcoma; Prostatic Neoplasms; Sarcoma; Medullary thyroid cancer (MTC); Carcinoma, Hepatocellular; Melanoma; Paraganglioma; Meningioma; Neoplasms, Germ Cell and Embryonal; Neoplasm Metastasis; Carcinoma, Non-Small-Cell Lung; Adenocarcinoma; Carcinoma, Endometrioid; Thyroid Cancer, Papillary; Brain Neoplasms; Carcinoma, Neuroendocrine; Sarcoma, Alveolar Soft Part; Lymphoma; Fallopian Tube Neoplasms; Brain metastases; Glioma; Endometrial Neoplasms; Carcinoma, Squamous Cell; Carcinoid Tumor; Glioblastoma; Skin Neoplasms; Neoplasms; Carcinoma, Papillary; Hepatoblastoma; Pheochromocytoma; Pain; Rejection of liver transplantation; Carcinoma, Renal Cell; Pancreatic neuroendocrine tumors | Details |
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| Insulin transdermal (Phosphagenics) | TPM-02 | Phase 2 Clinical | Phosphagenics | Diabetes Mellitus, Type 1; Diabetes Mellitus, Type 2; Primary Ovarian Insufficiency | Details |
| Insulin transdermal (Phosphagenics) | TPM-02 | Phase 2 Clinical | Phosphagenics | Diabetes Mellitus, Type 1; Diabetes Mellitus, Type 2; Primary Ovarian Insufficiency | Details |
This web search service is supported by Google Inc.
