 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!
 Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!  Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!
| 제품번호 | 종 | 제품 설명 | 구조 | 순도 | 특징 |
|---|---|---|---|---|---|
| SO1-H5148 | Human | Human SOD1 / Cu-Zn SOD Protein, His Tag (active enzyme) |  |
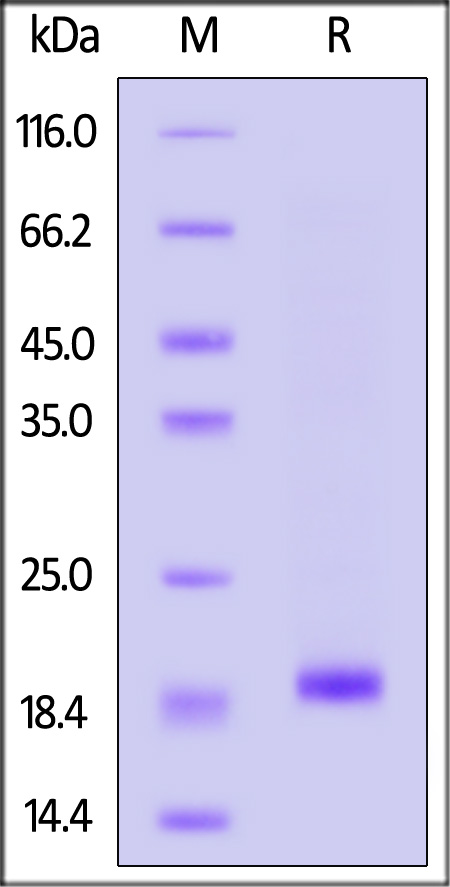
|
| Name | Research Code | Research Phase | Company | First Brand Name | First Approved Country | First Indication | First Approved Company | First Approved Date | Indications | Clinical Trials |
|---|---|---|---|---|---|---|---|---|---|---|
| Orgotein | Approved | Gt Biopharma Inc | Ontosein | Spain | Rheumatic Diseases; Cystitis; Radiation Injuries | null | 1996-01-01 | Cystitis; Radiation Injuries; Rheumatic Diseases | Details | |
| Cupric Sulfate/Manganese sulfate/Selenious acid/Zinc Sulfate | Approved | American Regent Inc | MULTRYS | United States | Dietary Supplement | American Regent Inc | 2020-07-02 | Dietary Supplement | Details | |
| Tofersen | ISIS-333611; BIIB-067; 99mTc-MAG3-BIIB067 | Approved | Ludwig Institute For Cancer Research, Ionis Pharmaceuticals Inc | QALSODY | United States | Amyotrophic Lateral Sclerosis | Biogen Ma Inc, Biogen Inc | 2023-04-25 | Amyotrophic Lateral Sclerosis | Details |
| Orgotein | Approved | Gt Biopharma Inc | Ontosein | Spain | Rheumatic Diseases; Cystitis; Radiation Injuries | null | 1996-01-01 | Cystitis; Radiation Injuries; Rheumatic Diseases | Details | |
| Cupric Sulfate/Manganese sulfate/Selenious acid/Zinc Sulfate | Approved | American Regent Inc | MULTRYS | United States | Dietary Supplement | American Regent Inc | 2020-07-02 | Dietary Supplement | Details | |
| Tofersen | ISIS-333611; BIIB-067; 99mTc-MAG3-BIIB067 | Approved | Ludwig Institute For Cancer Research, Ionis Pharmaceuticals Inc | QALSODY | United States | Amyotrophic Lateral Sclerosis | Biogen Ma Inc, Biogen Inc | 2023-04-25 | Amyotrophic Lateral Sclerosis | Details |
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| Superoxide dismutase (Ube Industries) | r-hSOD; FKU-871 | Phase 2 Clinical | Ube Industries Ltd, Astellas Pharma Inc | Myocardial Ischemia; Diabetic Neuropathies | Details |
| Otaplimastat | SP-8203 hydrochloride; SP8203 HCL; SP-8203 | Phase 2 Clinical | Shin Poong Pharmaceutical Co Ltd | Stroke | Details |
| BMX-010 | BMX-010; MnTE-2-PyP; MnTE-2-PyP5+ | Phase 2 Clinical | Duke University | Acne Vulgaris; Rosacea; Psoriasis; Dermatitis, Atopic | Details |
| APB-102 | APB-102; AMT-162 | Phase 2 Clinical | University Of Massachusetts Medical School | Amyotrophic Lateral Sclerosis | Details |
| Rucosopasem manganese | GC-4711 | Phase 1 Clinical | Galera Therapeutics Inc | Pancreatic Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| Bis-choline tetrathiomolybdate | ATN-224; WTX-101; ALXN-1840 | Phase 1 Clinical | Wilson Therapeutics | Hepatolenticular Degeneration; Multiple Myeloma; Prostatic Neoplasms; Melanoma | Details |
| NAS150 | AEOL-10150; NAS150; NAS-150 | Phase 1 Clinical | Aeolus Pharmaceuticals | Supranuclear Palsy, Progressive; Idiopathic Pulmonary Fibrosis; Neoplasms; Lung Injury | Details |
| NI-005 | AP-101 (AL-S Pharma); NI-005 | Phase 1 Clinical | Al-S Pharma Ag | Amyotrophic Lateral Sclerosis | Details |
| Superoxide dismutase (Ube Industries) | r-hSOD; FKU-871 | Phase 2 Clinical | Ube Industries Ltd, Astellas Pharma Inc | Myocardial Ischemia; Diabetic Neuropathies | Details |
| Otaplimastat | SP-8203 hydrochloride; SP8203 HCL; SP-8203 | Phase 2 Clinical | Shin Poong Pharmaceutical Co Ltd | Stroke | Details |
| BMX-010 | BMX-010; MnTE-2-PyP; MnTE-2-PyP5+ | Phase 2 Clinical | Duke University | Acne Vulgaris; Rosacea; Psoriasis; Dermatitis, Atopic | Details |
| APB-102 | APB-102; AMT-162 | Phase 2 Clinical | University Of Massachusetts Medical School | Amyotrophic Lateral Sclerosis | Details |
| Rucosopasem manganese | GC-4711 | Phase 1 Clinical | Galera Therapeutics Inc | Pancreatic Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| Bis-choline tetrathiomolybdate | ATN-224; WTX-101; ALXN-1840 | Phase 1 Clinical | Wilson Therapeutics | Hepatolenticular Degeneration; Multiple Myeloma; Prostatic Neoplasms; Melanoma | Details |
| NAS150 | AEOL-10150; NAS150; NAS-150 | Phase 1 Clinical | Aeolus Pharmaceuticals | Supranuclear Palsy, Progressive; Idiopathic Pulmonary Fibrosis; Neoplasms; Lung Injury | Details |
| NI-005 | AP-101 (AL-S Pharma); NI-005 | Phase 1 Clinical | Al-S Pharma Ag | Amyotrophic Lateral Sclerosis | Details |
This web search service is supported by Google Inc.
