 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!
 Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!  Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!
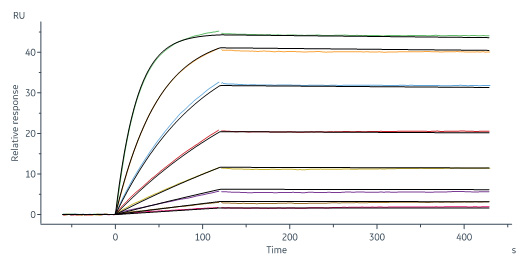
Human IL-1 RII, Fc Tag (Cat. No. IL2-H4256) captured on CM5 chip via Anti-human IgG Fc antibodies surface can bind Human IL-1 beta, His Tag (Cat. No. ILB-H51H3) with an affinity constant of 0.571 nM as determined in a SPR assay (Biacore 8K) (Routinely tested).
| Name | Research Code | Research Phase | Company | First Brand Name | First Approved Country | First Indication | First Approved Company | First Approved Date | Indications | Clinical Trials |
|---|---|---|---|---|---|---|---|---|---|---|
| Canakinumab | ACZ-885 | Approved | Novartis Pharma Ag | Ilaris | United States | Cryopyrin-Associated Periodic Syndromes; Muckle-Wells Syndrome (MWS) | Novartis Pharma Ag | 2009-06-17 | Gout; Hyperimmunoglobulin D syndrome; Mucocutaneous Lymph Node Syndrome; Cryopyrin-Associated Periodic Syndromes; Arthritis, Gouty; Cognitive Dysfunction; Sarcoidosis, Pulmonary; Alzheimer Disease; Urticaria; Muscular Dystrophy, Duchenne; Primary Myelofibrosis; Pulmonary Disease, Chronic Obstructive; Dry Eye Syndromes; Lung Neoplasms; Macular Degeneration; Carcinoma, Non-Small-Cell Lung; Anemia, Sickle Cell; Diabetes Mellitus; Behcet Syndrome; Aortic Aneurysm, Abdominal; Peripheral Arterial Disease; Diabetic Retinopathy; Thrombocythemia, Essential; Polycythemia Vera; Familial Mediterranean Fever; Prediabetic State; Diabetes Mellitus, Type 1; Hepatitis, Alcoholic; Schnitzler Syndrome; Atherosclerosis; Mevalonate Kinase Deficiency; Pyoderma Gangrenosum; Polyarticular Juvenile Idiopathic Arthritis; Diabetes Mellitus, Type 2; Anemia; Still's Disease, Adult-Onset; Arthritis, Rheumatoid; Small Cell Lung Carcinoma; Coronavirus Disease 2019 (COVID-19); Hereditary Autoinflammatory Diseases; Chronic Urticaria; Vasculit | Details |
| PG-201 | PG-201 | Approved | Viromed | Layla | South Korea | Osteoarthritis | Pmg Pharm Co Ltd | 2012-01-01 | Osteoarthritis, Knee; Osteoarthritis | Details |
| Edaravone/(+)-2-Decanol | SIM-071201; Y-2 | Approved | Jiangsu Simcere Pharmaceutical Co Ltd | Sanbexin, 三贝心, 三贝欣 | Mainland China | Stroke | Nanjing Simcere Dongyuan Pharmaceutical Co Ltd | 2020-07-29 | Intracranial Hemorrhages; Brain Infarction; Cerebral Hemorrhage; Stroke; Alzheimer Disease; Cognitive Dysfunction; Amyotrophic Lateral Sclerosis; Cognition Disorders | Details |
| Diacerein | KW-4800; SF-277; Art-50 | Approved | Cartivix, Fisiodar, Verboril, Art, 安必丁, Artrodar, Artrolyt | Metabolic Dysfunction-Associated Steatotic Liver Disease; Diabetes Mellitus, Type 2; Insulin Resistance; Coronavirus Disease 2019 (COVID-19); Epidermolysis Bullosa; Osteoarthritis, Knee; Diabetes Complications; Osteoarthritis; Obesity; Osteoarthritis, Hip; Diabetes Mellitus; Overweight | Details | |||||
| Andrographolide/Sodium Hydrogen Sulfite | Approved | Bacterial Infections | Details | |||||||
| Rilonacept | RGN-303; KPL-914 | Approved | Arcalyst | United States | Cryopyrin-Associated Periodic Syndromes | Kiniksa Pharmaceuticals Ltd | 2008-02-27 | Pericarditis; Inflammation; Scleroderma, Diffuse; Renal Insufficiency, Chronic; Gout; Urticaria; Cryopyrin-Associated Periodic Syndromes; Hearing Loss, Sensorineural; Genetic Diseases, Inborn; Diabetes Mellitus, Type 1; Arthritis, Juvenile; Deficiency of interleukin-1 receptor antagonist; Scleroderma, Systemic; Muckle-Wells Syndrome (MWS); Anemia; Schnitzler Syndrome; Familial Mediterranean Fever; Still's Disease, Adult-Onset | Details | |
| Thalidomide | NSC-66847; NSC-527179; K-17; VP-02; FPF-300; FPF300 | Approved | Celgene Corp | Talizer, Thalidomide Celgene, Thalidomide Pharmion, Synovir, Thalomid, Thaled | Mainland China | Leprosy, Lepromatous; Multiple Myeloma | Changzhou Pharmaceutical Factory | 1982-01-01 | HIV Wasting Syndrome; Angiodysplasia; Primary Myelofibrosis; Neuroectodermal Tumors, Primitive; Prostatitis; Colorectal Neoplasms; Osteosarcoma; Lymphoma, Mantle-Cell; Sarcoma, Ewing; Retinoblastoma; Erythema Nodosum; Drug Resistant Epilepsy; Xerostomia; Sarcoma; Pancreatitis, Chronic; Adenocarcinoma, Clear Cell; Lymphoma, Follicular; Arachnoiditis; Carcinoma, Adenosquamous; Gastrointestinal Hemorrhage; Cholangitis, Sclerosing; Prostatic Neoplasms; Pelvic Pain; Neoplasm Metastasis; Stomatitis; Burning Mouth Syndrome; Mycobacterium avium-intracellulare Infection; Amyotrophic Lateral Sclerosis; Melanoma; Myelodysplastic-Myeloproliferative Diseases; Carcinoma, Hepatocellular; Leukemia, Lymphocytic, Chronic, B-Cell; Vascular Malformations; Tuberculosis; Appendiceal Neoplasms; Lymphoma, Non-Hodgkin; Uterine Neoplasms; Anemia, Sideroblastic; Glioma; Leprosy, Lepromatous; Endometrial Neoplasms; Lung Neoplasms; Waldenstrom Macroglobulinemia; Kidney Neoplasms; Thalassemia; Carcinoid Tumor; Lupus Erythematosus, Discoid | Details |
| Canakinumab | ACZ-885 | Approved | Novartis Pharma Ag | Ilaris | United States | Cryopyrin-Associated Periodic Syndromes; Muckle-Wells Syndrome (MWS) | Novartis Pharma Ag | 2009-06-17 | Gout; Hyperimmunoglobulin D syndrome; Mucocutaneous Lymph Node Syndrome; Cryopyrin-Associated Periodic Syndromes; Arthritis, Gouty; Cognitive Dysfunction; Sarcoidosis, Pulmonary; Alzheimer Disease; Urticaria; Muscular Dystrophy, Duchenne; Primary Myelofibrosis; Pulmonary Disease, Chronic Obstructive; Dry Eye Syndromes; Lung Neoplasms; Macular Degeneration; Carcinoma, Non-Small-Cell Lung; Anemia, Sickle Cell; Diabetes Mellitus; Behcet Syndrome; Aortic Aneurysm, Abdominal; Peripheral Arterial Disease; Diabetic Retinopathy; Thrombocythemia, Essential; Polycythemia Vera; Familial Mediterranean Fever; Prediabetic State; Diabetes Mellitus, Type 1; Hepatitis, Alcoholic; Schnitzler Syndrome; Atherosclerosis; Mevalonate Kinase Deficiency; Pyoderma Gangrenosum; Polyarticular Juvenile Idiopathic Arthritis; Diabetes Mellitus, Type 2; Anemia; Still's Disease, Adult-Onset; Arthritis, Rheumatoid; Small Cell Lung Carcinoma; Coronavirus Disease 2019 (COVID-19); Hereditary Autoinflammatory Diseases; Chronic Urticaria; Vasculit | Details |
| PG-201 | PG-201 | Approved | Viromed | Layla | South Korea | Osteoarthritis | Pmg Pharm Co Ltd | 2012-01-01 | Osteoarthritis, Knee; Osteoarthritis | Details |
| Edaravone/(+)-2-Decanol | SIM-071201; Y-2 | Approved | Jiangsu Simcere Pharmaceutical Co Ltd | Sanbexin, 三贝心, 三贝欣 | Mainland China | Stroke | Nanjing Simcere Dongyuan Pharmaceutical Co Ltd | 2020-07-29 | Intracranial Hemorrhages; Brain Infarction; Cerebral Hemorrhage; Stroke; Alzheimer Disease; Cognitive Dysfunction; Amyotrophic Lateral Sclerosis; Cognition Disorders | Details |
| Diacerein | KW-4800; SF-277; Art-50 | Approved | Cartivix, Fisiodar, Verboril, Art, 安必丁, Artrodar, Artrolyt | Metabolic Dysfunction-Associated Steatotic Liver Disease; Diabetes Mellitus, Type 2; Insulin Resistance; Coronavirus Disease 2019 (COVID-19); Epidermolysis Bullosa; Osteoarthritis, Knee; Diabetes Complications; Osteoarthritis; Obesity; Osteoarthritis, Hip; Diabetes Mellitus; Overweight | Details | |||||
| Andrographolide/Sodium Hydrogen Sulfite | Approved | Bacterial Infections | Details | |||||||
| Rilonacept | RGN-303; KPL-914 | Approved | Arcalyst | United States | Cryopyrin-Associated Periodic Syndromes | Kiniksa Pharmaceuticals Ltd | 2008-02-27 | Pericarditis; Inflammation; Scleroderma, Diffuse; Renal Insufficiency, Chronic; Gout; Urticaria; Cryopyrin-Associated Periodic Syndromes; Hearing Loss, Sensorineural; Genetic Diseases, Inborn; Diabetes Mellitus, Type 1; Arthritis, Juvenile; Deficiency of interleukin-1 receptor antagonist; Scleroderma, Systemic; Muckle-Wells Syndrome (MWS); Anemia; Schnitzler Syndrome; Familial Mediterranean Fever; Still's Disease, Adult-Onset | Details | |
| Thalidomide | NSC-66847; NSC-527179; K-17; VP-02; FPF-300; FPF300 | Approved | Celgene Corp | Talizer, Thalidomide Celgene, Thalidomide Pharmion, Synovir, Thalomid, Thaled | Mainland China | Leprosy, Lepromatous; Multiple Myeloma | Changzhou Pharmaceutical Factory | 1982-01-01 | HIV Wasting Syndrome; Angiodysplasia; Primary Myelofibrosis; Neuroectodermal Tumors, Primitive; Prostatitis; Colorectal Neoplasms; Osteosarcoma; Lymphoma, Mantle-Cell; Sarcoma, Ewing; Retinoblastoma; Erythema Nodosum; Drug Resistant Epilepsy; Xerostomia; Sarcoma; Pancreatitis, Chronic; Adenocarcinoma, Clear Cell; Lymphoma, Follicular; Arachnoiditis; Carcinoma, Adenosquamous; Gastrointestinal Hemorrhage; Cholangitis, Sclerosing; Prostatic Neoplasms; Pelvic Pain; Neoplasm Metastasis; Stomatitis; Burning Mouth Syndrome; Mycobacterium avium-intracellulare Infection; Amyotrophic Lateral Sclerosis; Melanoma; Myelodysplastic-Myeloproliferative Diseases; Carcinoma, Hepatocellular; Leukemia, Lymphocytic, Chronic, B-Cell; Vascular Malformations; Tuberculosis; Appendiceal Neoplasms; Lymphoma, Non-Hodgkin; Uterine Neoplasms; Anemia, Sideroblastic; Glioma; Leprosy, Lepromatous; Endometrial Neoplasms; Lung Neoplasms; Waldenstrom Macroglobulinemia; Kidney Neoplasms; Thalassemia; Carcinoid Tumor; Lupus Erythematosus, Discoid | Details |
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| Ensereptide | PXL-01 | Phase 3 Clinical | Promore Pharma | Post-surgical adhesions; Tissue Adhesions; Cicatrix | Details |
| Efprezimod alfa | CD24-Fc; HAS-CD24; CD24-Fc-IgG; MK-7110; MK7110 | Phase 3 Clinical | Oncoimmune Inc | Solid tumours; Hematopoietic stem cell transplantation (HSCT); Leukemia; HIV Infections; Graft vs Host Disease; Myelodysplastic Syndromes; Coronavirus Disease 2019 (COVID-19); Precursor Cell Lymphoblastic Leukemia-Lymphoma; Dyslipidemias; Leukemia, Myeloid, Acute; Melanoma | Details |
| Goflikicept | RPH-104 | Phase 3 Clinical | Trpharm | Still's Disease, Adult-Onset; Familial Mediterranean Fever; Myocardial Infarction; Schnitzler Syndrome; ST Elevation Myocardial Infarction; Coronavirus Disease 2019 (COVID-19); Pericarditis; Gout | Details |
| Recombinant humanized monoclonal antibody antiIL-1β(Cp Guojian) | SSGJ-613 | Phase 3 Clinical | Sunshine GuoJian Pharmaceutical (Shanghai) Co Ltd | Tumor necrosis factor receptor associated periodic syndrome; Arthritis, Juvenile; Arthritis, Gouty; Gout; Inflammation | Details |
| MAS-825 | MAS-825; MAS825 | Phase 3 Clinical | Novartis Pharmaceuticals Corp | Enterocolitis; Coronavirus Disease 2019 (COVID-19); Coronary Disease; Autoimmune Lymphoproliferative Syndrome | Details |
| lutikizumab | ABT-981 | Phase 2 Clinical | Abbvie Inc | Osteoarthritis, Knee; Colitis, Ulcerative; Hidradenitis Suppurativa; Osteoarthritis | Details |
| CU-06 | CU-06-EYE; CU-06-IBD; CU-061004; Sac-1004; CU-06-ONCO; CU-06-HAE; CU06-1004 | Phase 2 Clinical | Curacle Co Ltd | Myocardial Infarction; Neoplasms; Lung Diseases; Stroke; Diabetic macular oedema; Colitis, Ulcerative; Diabetic Retinopathy; Crohn Disease; Macular Degeneration; Angioedemas, Hereditary | Details |
| IL1-beta receptor antagonist Therapy(Hamlet BioPharma) | Phase 2 Clinical | Hamlet BioPharma AB | Cystitis; Pain | Details | |
| Diacerein (TWi Biotechnology/Castle Creek Pharmaceuticals) | AC-201; AC-201 Controlled release tablet; AC-201 CR; AC-203; CCP 020; CCP-020 | Phase 2 Clinical | Twi Biotechnology Inc | Diabetes Mellitus, Type 2; Epidermolysis Bullosa; Epidermolysis Bullosa Simplex; Pemphigoid, Bullous; Epidermolysis Bullosa, Junctional; Arthritis; Gout; Epidermolysis Bullosa Dystrophica | Details |
| Gevokizumab | XMA-005.2; AB-7; XOMA-052; S-78989; VPM-087; XMA-0052 | Phase 1 Clinical | Xoma Corp | Colonic Neoplasms; Behcet Syndrome; Panuveitis; Labyrinth Diseases; Osteoarthritis; Uveitis; Colorectal Neoplasms; Cryopyrin-Associated Periodic Syndromes; Hereditary Autoinflammatory Diseases; Diabetes Mellitus, Type 1; Arthritis, Rheumatoid; Pyoderma Gangrenosum; Stomach Neoplasms; Carcinoma, Renal Cell; Esophageal Neoplasms; Diabetes Mellitus, Type 2; Acne Vulgaris | Details |
| DLX-2323 | DLX-2323 | Phase 1 Clinical | Delenex Therapeutics Ag | Inflammation | Details |
| Tavo-103 | Tavo103; Tavo-103; TAVO-103A; TAVO103A | Phase 1 Clinical | Tavotek Biotherapeutics (Hong Kong) Ltd | Details | |
| FL-101 | FL-101 | Phase 1 Clinical | Flame Biosciences Inc | Urinary Bladder Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| AK-114 | AK-114; AK114 | Phase 1 Clinical | Zhongshan Akeso Biopharma Co Ltd | Solid tumours; Neoplasms; Inflammation; Neoplasm Metastasis | Details |
| Ensereptide | PXL-01 | Phase 3 Clinical | Promore Pharma | Post-surgical adhesions; Tissue Adhesions; Cicatrix | Details |
| Efprezimod alfa | CD24-Fc; HAS-CD24; CD24-Fc-IgG; MK-7110; MK7110 | Phase 3 Clinical | Oncoimmune Inc | Solid tumours; Hematopoietic stem cell transplantation (HSCT); Leukemia; HIV Infections; Graft vs Host Disease; Myelodysplastic Syndromes; Coronavirus Disease 2019 (COVID-19); Precursor Cell Lymphoblastic Leukemia-Lymphoma; Dyslipidemias; Leukemia, Myeloid, Acute; Melanoma | Details |
| Goflikicept | RPH-104 | Phase 3 Clinical | Trpharm | Still's Disease, Adult-Onset; Familial Mediterranean Fever; Myocardial Infarction; Schnitzler Syndrome; ST Elevation Myocardial Infarction; Coronavirus Disease 2019 (COVID-19); Pericarditis; Gout | Details |
| Recombinant humanized monoclonal antibody antiIL-1β(Cp Guojian) | SSGJ-613 | Phase 3 Clinical | Sunshine GuoJian Pharmaceutical (Shanghai) Co Ltd | Tumor necrosis factor receptor associated periodic syndrome; Arthritis, Juvenile; Arthritis, Gouty; Gout; Inflammation | Details |
| MAS-825 | MAS-825; MAS825 | Phase 3 Clinical | Novartis Pharmaceuticals Corp | Enterocolitis; Coronavirus Disease 2019 (COVID-19); Coronary Disease; Autoimmune Lymphoproliferative Syndrome | Details |
| lutikizumab | ABT-981 | Phase 2 Clinical | Abbvie Inc | Osteoarthritis, Knee; Colitis, Ulcerative; Hidradenitis Suppurativa; Osteoarthritis | Details |
| CU-06 | CU-06-EYE; CU-06-IBD; CU-061004; Sac-1004; CU-06-ONCO; CU-06-HAE; CU06-1004 | Phase 2 Clinical | Curacle Co Ltd | Myocardial Infarction; Neoplasms; Lung Diseases; Stroke; Diabetic macular oedema; Colitis, Ulcerative; Diabetic Retinopathy; Crohn Disease; Macular Degeneration; Angioedemas, Hereditary | Details |
| IL1-beta receptor antagonist Therapy(Hamlet BioPharma) | Phase 2 Clinical | Hamlet BioPharma AB | Cystitis; Pain | Details | |
| Diacerein (TWi Biotechnology/Castle Creek Pharmaceuticals) | AC-201; AC-201 Controlled release tablet; AC-201 CR; AC-203; CCP 020; CCP-020 | Phase 2 Clinical | Twi Biotechnology Inc | Diabetes Mellitus, Type 2; Epidermolysis Bullosa; Epidermolysis Bullosa Simplex; Pemphigoid, Bullous; Epidermolysis Bullosa, Junctional; Arthritis; Gout; Epidermolysis Bullosa Dystrophica | Details |
| Gevokizumab | XMA-005.2; AB-7; XOMA-052; S-78989; VPM-087; XMA-0052 | Phase 1 Clinical | Xoma Corp | Colonic Neoplasms; Behcet Syndrome; Panuveitis; Labyrinth Diseases; Osteoarthritis; Uveitis; Colorectal Neoplasms; Cryopyrin-Associated Periodic Syndromes; Hereditary Autoinflammatory Diseases; Diabetes Mellitus, Type 1; Arthritis, Rheumatoid; Pyoderma Gangrenosum; Stomach Neoplasms; Carcinoma, Renal Cell; Esophageal Neoplasms; Diabetes Mellitus, Type 2; Acne Vulgaris | Details |
| DLX-2323 | DLX-2323 | Phase 1 Clinical | Delenex Therapeutics Ag | Inflammation | Details |
| Tavo-103 | Tavo103; Tavo-103; TAVO-103A; TAVO103A | Phase 1 Clinical | Tavotek Biotherapeutics (Hong Kong) Ltd | Details | |
| FL-101 | FL-101 | Phase 1 Clinical | Flame Biosciences Inc | Urinary Bladder Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| AK-114 | AK-114; AK114 | Phase 1 Clinical | Zhongshan Akeso Biopharma Co Ltd | Solid tumours; Neoplasms; Inflammation; Neoplasm Metastasis | Details |
This web search service is supported by Google Inc.
