 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!
 Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!  Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!

Expression analysis of human ErbB3 on HEK293/Human ErbB3 Stable Cell Line by FACS.
Cell surface staining was performed on HEK293/Human ErbB3 Stable Cell Line or negative control cell using PE-labeled anti-human ErbB3 antibody.
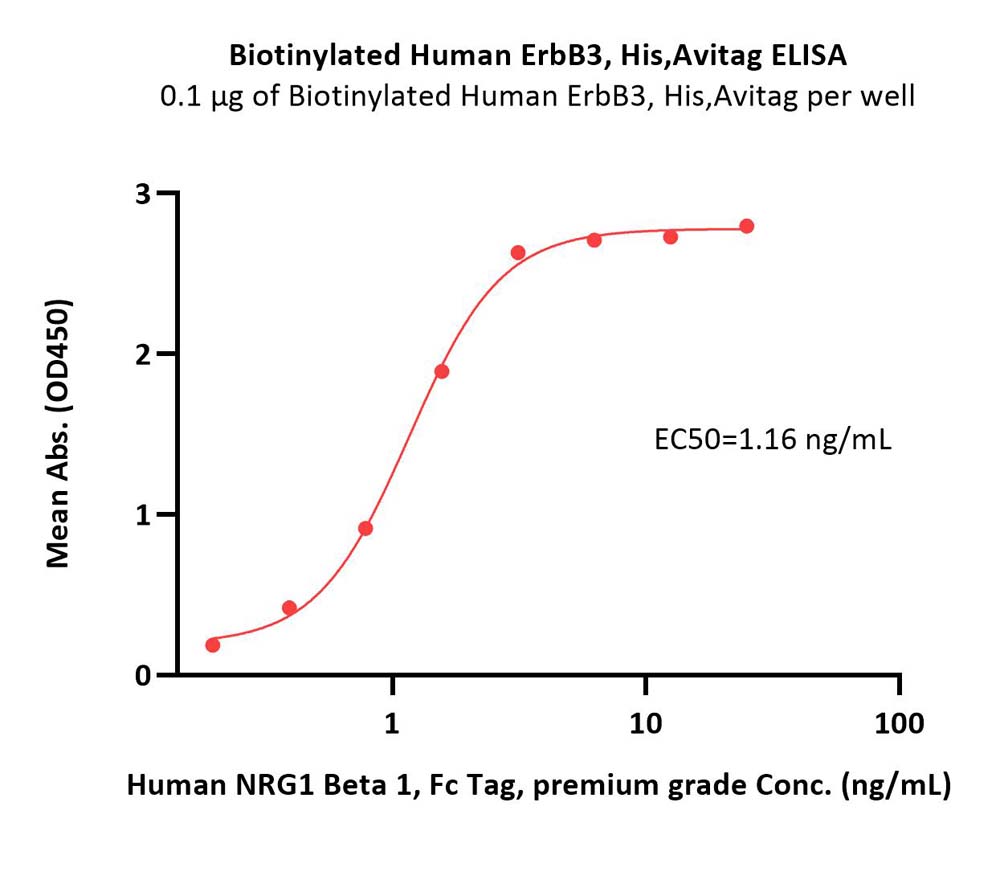
Immobilized Biotinylated Human ErbB3, His,Avitag (Cat. No. ER3-H82E6) at 1 μg/mL (100 μL/well) on streptavidin (Cat. No. STN-N5116) precoated (0.5 μg/well) plate can bind Human NRG1 Beta 1, Fc Tag, premium grade (Cat. No. NR1-H5268) with a linear range of 0.2-3 ng/mL (QC tested).
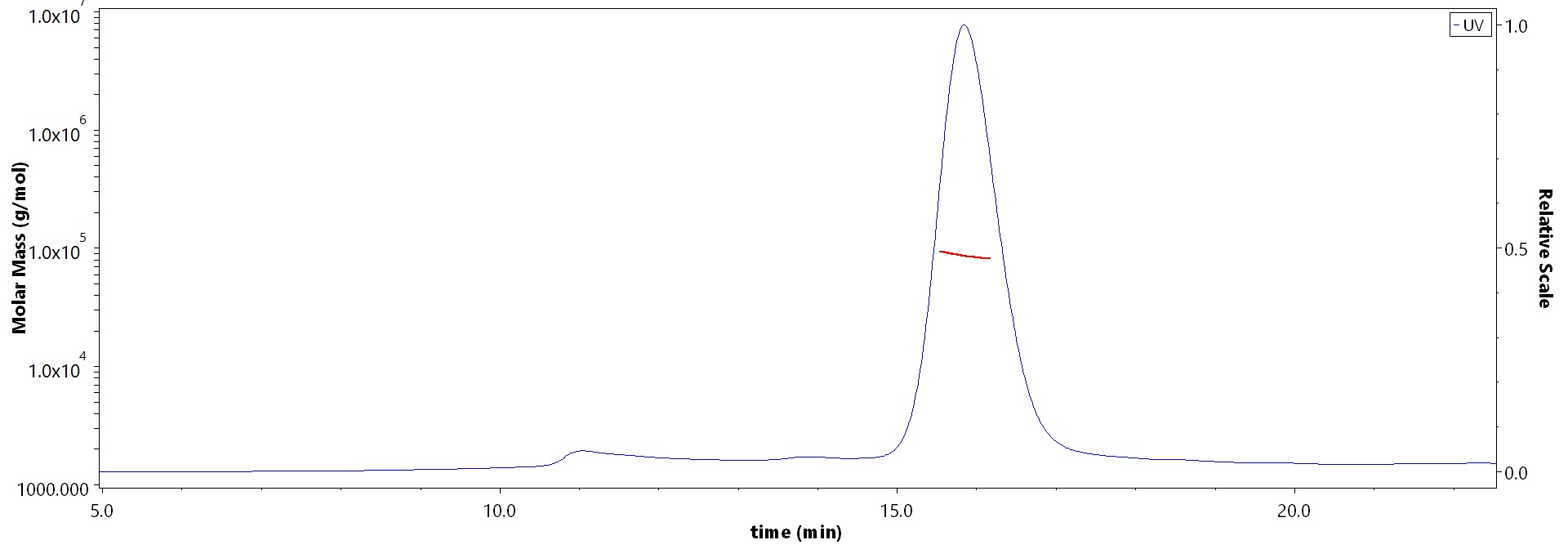
The purity of Mouse ErbB3, His Tag (Cat. No. ER3-M52H5) is more than 85% and the molecular weight of this protein is around 75-110 kDa verified by SEC-MALS.
| Name | Research Code | Research Phase | Company | First Brand Name | First Approved Country | First Indication | First Approved Company | First Approved Date | Indications | Clinical Trials |
|---|---|---|---|---|---|---|---|---|---|---|
| Osimertinib Mesylate | AZD-9291; RDL94R2A16; AZD-9291 Mesylate; AZD9291 | Approved | Astrazeneca Plc | 泰瑞沙, Tagrisso | United States | Carcinoma, Non-Small-Cell Lung | Astrazeneca Pharmaceutical Co Ltd | 2015-11-13 | Uterine Neoplasms; Urinary Bladder Neoplasms; Multiple Myeloma; Prostatic Neoplasms; Cholangiocarcinoma; Breast Neoplasms; Meningeal Neoplasms; Colorectal Neoplasms; Lung Neoplasms; Brain metastases; Adenocarcinoma of Lung; Endometrial Neoplasms; Thyroid Neoplasms; Carcinoma, Squamous Cell; Lymphoma; Glioma; Carcinoma, Non-Small-Cell Lung; Uterine Cervical Neoplasms; Adenocarcinoma; Melanoma; Esophageal Neoplasms; Liver Neoplasms; Head and Neck Neoplasms; Neoplasm, Residual; Ovarian Neoplasms; Hematologic Neoplasms; Carcinoma, Renal Cell; Stomach Neoplasms; Rectal Neoplasms; Squamous Cell Carcinoma of Head and Neck; Solid tumours; Neoplasms; Glioblastoma; Colonic Neoplasms; Carcinoma, Transitional Cell; Carcinoma, Ovarian Epithelial; Skin Neoplasms; Small Cell Lung Carcinoma; Pancreatic Neoplasms | Details |
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| Izalontamab | SI-B001; SI-1X6.4 | Phase 3 Clinical | Solid tumours; Head and Neck Neoplasms; Squamous Cell Carcinoma of Head and Neck; Stomach Neoplasms; Neoplasms, Glandular and Epithelial; Triple Negative Breast Neoplasms; Digestive System Neoplasms; Colorectal Neoplasms; Carcinoma, Squamous Cell; Esophageal Squamous Cell Carcinoma; Carcinoma, Non-Small-Cell Lung | Details | |
| Izalontamab brengitecan | BL-B01D1 | Phase 3 Clinical | SystImmune | Nasopharyngeal Carcinoma; Gastrointestinal Neoplasms; Uterine Cervical Neoplasms; Carcinoma, Non-Small-Cell Lung; Metastatic breast cancer; Lung Neoplasms; Esophageal Squamous Cell Carcinoma; Colorectal Neoplasms; Urologic Neoplasms; Breast Neoplasms; Solid tumours; Digestive System Neoplasms; Small Cell Lung Carcinoma; Neoplasms; Carcinoma, Transitional Cell; Squamous Cell Carcinoma of Head and Neck; Stomach Neoplasms; Esophageal Neoplasms; Neoplasms, Fibroepithelial | Details |
| Nezutatug | HMBD-001 | Phase 2 Clinical | Hummingbird Bioscience | Ovarian Neoplasms; Solid tumours; Squamous Cell Carcinoma of Head and Neck; Esophageal Neoplasms; Stomach Neoplasms; Triple Negative Breast Neoplasms; Pancreatic Neoplasms; Prostatic Neoplasms, Castration-Resistant; Urinary Bladder Neoplasms; Colorectal Neoplasms; Endometrial Neoplasms; Carcinoma, Non-Small-Cell Lung; Melanoma; Carcinoma, Hepatocellular; Neoplasm Metastasis; Uterine Cervical Neoplasms | Details |
| Sapitinib | AZD-8931 | Phase 2 Clinical | Astrazeneca Plc | Solid tumours; Stomach Neoplasms; Esophageal Neoplasms; Neoplasms; Breast Neoplasms; Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung; Neoplasm Metastasis | Details |
| Seribantumab | MM-121; SAR-256212; 1N3L70MDFX (UNII code) | Phase 2 Clinical | Merrimack Pharmaceuticals Inc | Prostatic Neoplasms; Adenocarcinoma; Carcinoma, Endometrioid; Carcinoma, Non-Small-Cell Lung; Neoplasm Metastasis; Lung Neoplasms; Gallbladder Neoplasms; Uterine Neoplasms; Fallopian Tube Neoplasms; Bile Duct Neoplasms; Colorectal Neoplasms; Peritoneal Neoplasms; Sarcoma; Cholangiocarcinoma; Ovarian Neoplasms; Breast Neoplasms; Urinary Bladder Neoplasms; Pancreatic Neoplasms; Carcinoma, Ovarian Epithelial; Triple Negative Breast Neoplasms; Small Cell Lung Carcinoma; Neoplasms; Esophageal Neoplasms; Squamous Cell Carcinoma of Head and Neck; Head and Neck Neoplasms; Kidney Neoplasms; Solid tumours | Details |
| Tesevatinib | XL-647; KD-020; KD-019; EXEL-7647 | Phase 2 Clinical | Exelixis Inc | Glioblastoma; Neoplasms; Brain Neoplasms; Breast Neoplasms; Polycystic Kidney, Autosomal Dominant; Brain metastases; Polycystic Kidney, Autosomal Recessive; Neoplasm Metastasis; Carcinoma, Non-Small-Cell Lung | Details |
| Allitinib Tosylate | AST-6; ALS-1306; AST-1306 | Phase 2 Clinical | Shanghai Allist Pharmaceutical Technology Co Ltd | Breast Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| Zenocutuzumab | MCLA-128 | Phase 2 Clinical | Merus Nv | Solid tumours; Pancreatic Neoplasms; Prostatic Neoplasms, Castration-Resistant; Metastatic breast cancer; Carcinoma, Non-Small-Cell Lung | Details |
| 89Zr-Patritumab deruxtecan | Phase 2 Clinical | Daiichi Sankyo Co Ltd, The Netherlands Cancer Institute, Daiichi Sankyo Inc | Carcinoma, Non-Small-Cell Lung | Details | |
| IBI-133 | IBI133; IBI-133 | Phase 2 Clinical | Innovent Biologics(Suzhou) Co Ltd | Solid tumours | Details |
| DB-1310 | DB-1310 | Phase 2 Clinical | Solid tumours; Neoplasm Metastasis | Details | |
| SIBP-03 | SIBP-03 | Phase 2 Clinical | Shanghai Institute Of Biological Products Co Ltd | Squamous Cell Carcinoma of Head and Neck; Neoplasms | Details |
| SHR-A2009 | SHR-A2009 | Phase 2 Clinical | Suzhou Suncadia Biopharmaceuticals Co Ltd | Solid tumours; Breast Neoplasms | Details |
| Recombinant Human Neuromodulin 1-Anti-HER3 Antibody Fusion Protein(Salubris) | JK07; SAL-007 | Phase 2 Clinical | Shenzhen Salubris Pharmaceuticals Co Ltd | Heart Failure | Details |
| Elgemtumab | NOV-6; LJM-716 | Phase 1 Clinical | Novartis Pharma Ag, Morphosys Ag | Head and Neck Neoplasms; Squamous Cell Carcinoma of Head and Neck; Stomach Neoplasms; Neoplasms; Breast Neoplasms; Esophageal Squamous Cell Carcinoma | Details |
| MP-0274 | DARPin-41; SPA-28; CME-114; CME-115; CME-118; CME-119; MP-0274 | Phase 1 Clinical | Molecular Partners Ag | Neoplasms | Details |
| AV-203 | AV-203; CAN-017 | Phase 1 Clinical | Aveo | Solid tumours; Neoplasms | Details |
| Barecetamab | ISU-104 | Phase 1 Clinical | Isu Abxis Co Ltd | Solid tumours | Details |
| Sirotinib Maleate | XZP-5491 | Phase 1 Clinical | Shandong Xuanzhu Pharmaceutical Technology Co Ltd | Stomach Neoplasms; Carcinoma, Squamous Cell; Carcinoma, Non-Small-Cell Lung | Details |
| SYS-6023 | SYS-6023; SYS6023 | Phase 1 Clinical | Jushi Biopharmaceutical Co Ltd | Solid tumours | Details |
| AMT-562 | AMT-562 | Phase 1 Clinical | Multitude Therapeutics Inc | Solid tumours; Stomach Neoplasms; Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| SIBP-A13 | SIBP-A13 | Phase 1 Clinical | Shanghai Institute Of Biological Products Co Ltd | Head and Neck Neoplasms; Solid tumours; Metastatic breast cancer; Carcinoma, Non-Small-Cell Lung | Details |
| Recombinant human ErbB3 fragment vaccine (Zensun) | rhErbB3-f | Phase 1 Clinical | Zensun (Shanghai) Sci&Tech Co Ltd | Neoplasms | Details |
| Recombinat humanized HER3-targeting antibody | Phase 1 Clinical | Shanghai Institute Of Biological Products Co Ltd | Solid tumours | Details |
This web search service is supported by Google Inc.
