 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!
 Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!  Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!
| 제품번호 | 종 | 제품 설명 | 구조 | 순도 | 특징 |
|---|---|---|---|---|---|
| CD9-H82E4 | Human | Biotinylated Human CD109 / CPAMD7 Protein, His,Avitag™ (MALS & SPR verified) |  |
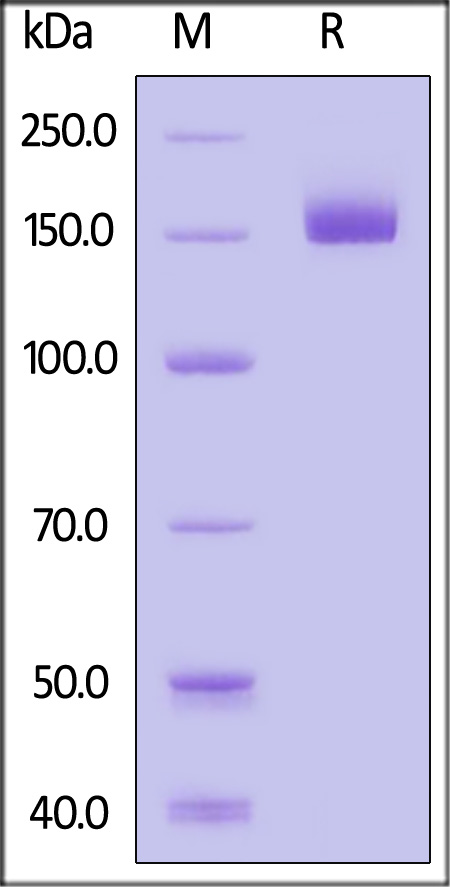
|
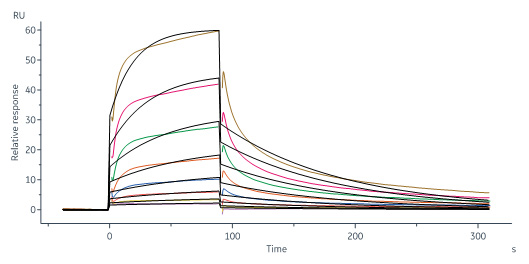
Biotinylated Human CD109, His,Avitag (Cat. No. CD9-H82E4) immobilized on CM5 Chip can bind Biotinylated Human TGF-Beta 1, Avitag (Cat. No. TG1-H8217) with an affinity constant of 0.248 μM as determined in a SPR assay (Biacore 8K) (QC tested).

The purity of Biotinylated Human CD109, His,Avitag (Cat. No. CD9-H82E4) is more than 85% and the molecular weight of this protein is around 150-180 kDa verified by SEC-MALS.
| Name | Research Code | Research Phase | Company | First Brand Name | First Approved Country | First Indication | First Approved Company | First Approved Date | Indications | Clinical Trials |
|---|---|---|---|---|---|---|---|---|---|---|
| Clofarabine | CAFdA; JC0707; GZ-393590; SAR-393590; 2-Cl-2-F-araA | Approved | Genzyme Corp | Clolar, Evoltra, Ivozall, Clofarex | United States | Precursor Cell Lymphoblastic Leukemia-Lymphoma | Genzyme Corp | 2004-12-28 | Lymphoma, Large B-Cell, Diffuse; Leukemia, Lymphocytic, Chronic, B-Cell; Lymphoma, Large-Cell, Anaplastic; Lymphoma, T-Cell; Waldenstrom Macroglobulinemia; Lymphoma, Non-Hodgkin; Lymphoma; Leukemia, Myeloid, Acute; Lymphoproliferative Disorders; Lymphoma, Mantle-Cell; Lymphoma, Follicular; Histiocytosis, Langerhans-Cell; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Multiple Myeloma; Hematologic Neoplasms; Neoplasms; Myelodysplastic Syndromes; Leukemia, Myelomonocytic, Chronic; Leukemia, Lymphoid; Lymphoma, B-Cell, Marginal Zone; Lymphoma, B-Cell; Lymphoma, T-Cell, Peripheral; Intestinal Neoplasms; Solid tumours; Leukemia, Myelogenous, Chronic; Leukemia, Myeloid; Leukemia | Details |
| Nelarabine | 506U; 506-U78; GW-506U; GW-506U78; GI-262250X; NSC-686673 | Approved | Novartis Pharma Ag, Glaxosmithkline Plc | Arranon, ArranonG, Atriance | United States | Precursor T-Cell Lymphoblastic Leukemia-Lymphoma | Sandoz | 2005-10-28 | Sezary Syndrome; Mycosis Fungoides; Leukemia, Lymphocytic, Chronic, B-Cell; Leukemia, T-Cell; Lymphoma, T-Cell, Cutaneous; Lymphoma, Large-Cell, Anaplastic; Lymphoma, Non-Hodgkin; Waldenstrom Macroglobulinemia; Lymphoma; Lymphoma, T-Cell; Leukemia; Lymphoma, Follicular; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Precursor T-Cell Lymphoblastic Leukemia-Lymphoma; Multiple Myeloma; Myeloproliferative Disorders; Myelodysplastic Syndromes; Plasmacytoma; Lymphoma, B-Cell, Marginal Zone | Details |
| Clofarabine | CAFdA; JC0707; GZ-393590; SAR-393590; 2-Cl-2-F-araA | Approved | Genzyme Corp | Clolar, Evoltra, Ivozall, Clofarex | United States | Precursor Cell Lymphoblastic Leukemia-Lymphoma | Genzyme Corp | 2004-12-28 | Lymphoma, Large B-Cell, Diffuse; Leukemia, Lymphocytic, Chronic, B-Cell; Lymphoma, Large-Cell, Anaplastic; Lymphoma, T-Cell; Waldenstrom Macroglobulinemia; Lymphoma, Non-Hodgkin; Lymphoma; Leukemia, Myeloid, Acute; Lymphoproliferative Disorders; Lymphoma, Mantle-Cell; Lymphoma, Follicular; Histiocytosis, Langerhans-Cell; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Multiple Myeloma; Hematologic Neoplasms; Neoplasms; Myelodysplastic Syndromes; Leukemia, Myelomonocytic, Chronic; Leukemia, Lymphoid; Lymphoma, B-Cell, Marginal Zone; Lymphoma, B-Cell; Lymphoma, T-Cell, Peripheral; Intestinal Neoplasms; Solid tumours; Leukemia, Myelogenous, Chronic; Leukemia, Myeloid; Leukemia | Details |
| Nelarabine | 506U; 506-U78; GW-506U; GW-506U78; GI-262250X; NSC-686673 | Approved | Novartis Pharma Ag, Glaxosmithkline Plc | Arranon, ArranonG, Atriance | United States | Precursor T-Cell Lymphoblastic Leukemia-Lymphoma | Sandoz | 2005-10-28 | Sezary Syndrome; Mycosis Fungoides; Leukemia, Lymphocytic, Chronic, B-Cell; Leukemia, T-Cell; Lymphoma, T-Cell, Cutaneous; Lymphoma, Large-Cell, Anaplastic; Lymphoma, Non-Hodgkin; Waldenstrom Macroglobulinemia; Lymphoma; Lymphoma, T-Cell; Leukemia; Lymphoma, Follicular; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Precursor T-Cell Lymphoblastic Leukemia-Lymphoma; Multiple Myeloma; Myeloproliferative Disorders; Myelodysplastic Syndromes; Plasmacytoma; Lymphoma, B-Cell, Marginal Zone | Details |
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| Clofarabine (Boyen) | BY-101 | Phase 3 Clinical | Boyen Therapeutics Inc | Leukemia, Myeloid, Acute | Details |
| Alkasar-18 | NOAC; Alkasar-18; N4-octadecyl-ara-C | Phase 2 Clinical | University of Zurich | Cerebral Hemorrhage; Neoplasms; Atrial Fibrillation | Details |
| Sapacitabine | CS-682; CYC-682 | Phase 2 Clinical | Daiichi Sankyo Co Ltd | Leukemia; Myelodysplastic Syndromes; Breast Neoplasms; Leukemia, Myeloid, Acute; Lymphoma, T-Cell, Cutaneous; Carcinoma, Non-Small-Cell Lung | Details |
| Clofarabine (Boyen) | BY-101 | Phase 3 Clinical | Boyen Therapeutics Inc | Leukemia, Myeloid, Acute | Details |
| Alkasar-18 | NOAC; Alkasar-18; N4-octadecyl-ara-C | Phase 2 Clinical | University of Zurich | Cerebral Hemorrhage; Neoplasms; Atrial Fibrillation | Details |
| Sapacitabine | CS-682; CYC-682 | Phase 2 Clinical | Daiichi Sankyo Co Ltd | Leukemia; Myelodysplastic Syndromes; Breast Neoplasms; Leukemia, Myeloid, Acute; Lymphoma, T-Cell, Cutaneous; Carcinoma, Non-Small-Cell Lung | Details |
This web search service is supported by Google Inc.
