Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!
 Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!  Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!
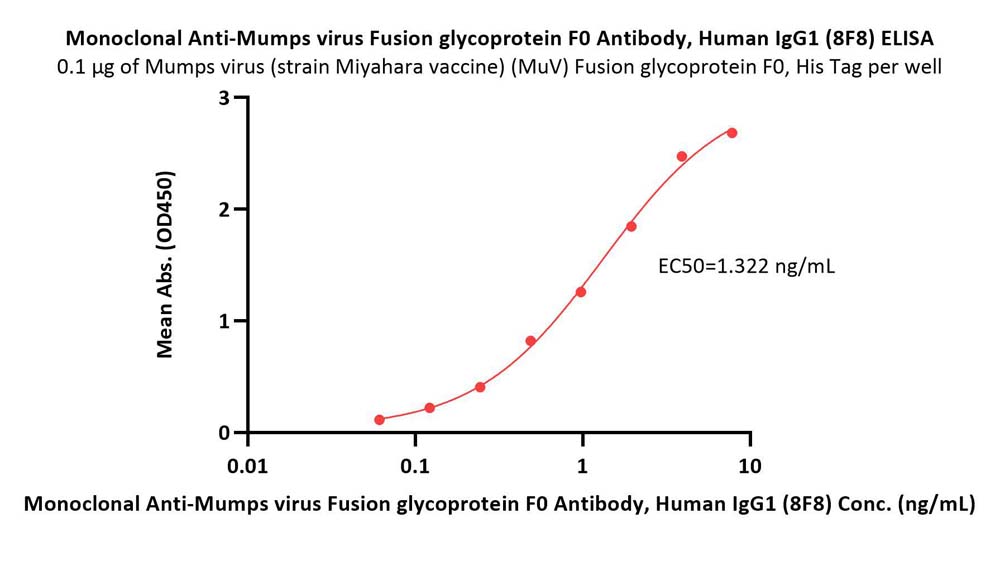
Immobilized Mumps virus (strain Miyahara vaccine) (MuV) Fusion glycoprotein F0, His Tag (Cat. No. RSF-V52H4) at 1 μg/mL (100 μL/well) can bind Monoclonal Anti-Mumps virus Fusion glycoprotein F0 Antibody, Human IgG1 (8F8) (Cat. No. RSF-MY2093) with a linear range of 0.06-2 ng/mL (QC tested).
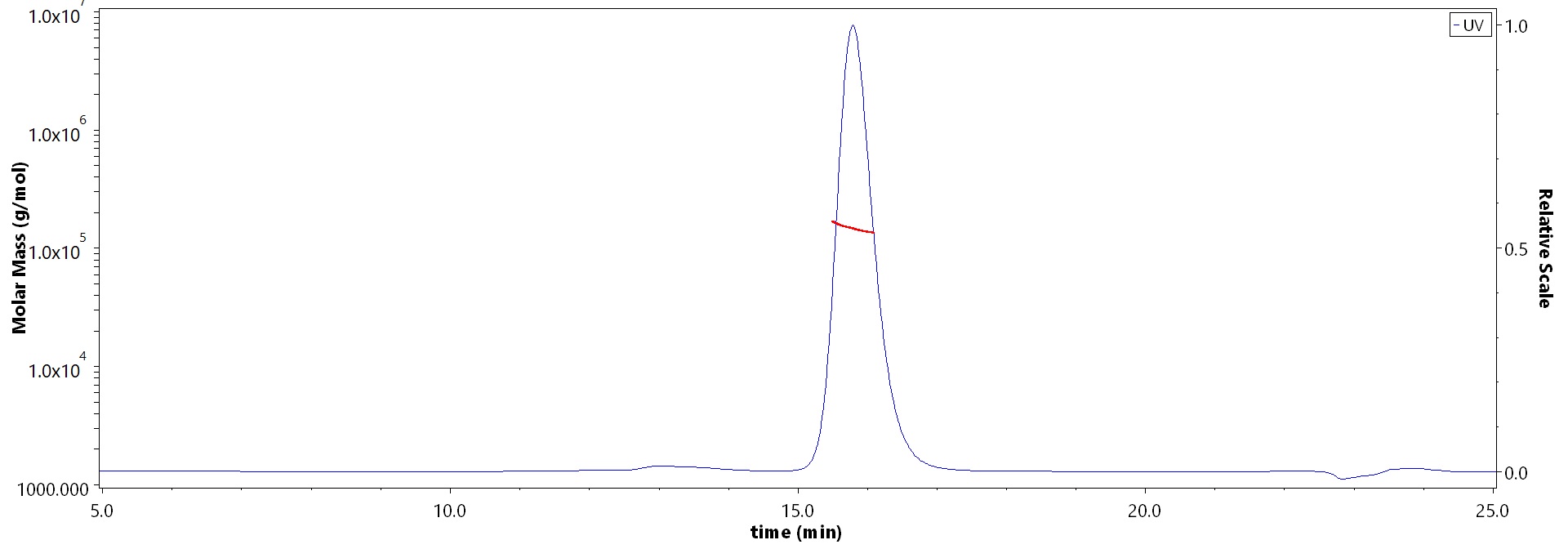
The purity of Monoclonal Anti-Mumps virus Fusion glycoprotein F0 Antibody, Human IgG1 (5A3) (Cat. No. RSF-MY2092) is more than 90% and the molecular weight of this protein is around 135-160 kDa verified by SEC-MALS.
This web search service is supported by Google Inc.