



























 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!
 Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!  Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!
> Insights > Solutions for AAV Research & Development: AAV Titration and Anti-AAV Antibody ELISA Kits Adeno-associated virus (AAV) vectors have emerged as a pivotal tool in gene therapy, offering a promising delivery system in treating genetic disorders. These small, non-pathogenic viruses belong to the Parvoviridae family and are characterized by their capsid proteins: VP1, VP2, and VP3. Mutations in these capsid proteins give rise to various AAV serotypes, with over ten well-characterized types (AAV1 to AAV10) exhibiting distinct tissue tropisms and immune profiles. This diversity provides researchers with a versatile toolkit for targeting specific tissues while minimizing immunogenicity.
The popularity of AAV vectors in gene therapy stems from their exceptional biosafety profile, stable gene expression, and low immunogenicity. These attributes, coupled with their broad host range and strong tissue specificity, have led to their widespread adoption, with AAV-based delivery systems now accounting for over 70% of gene therapy drugs. The significant market potential and growing demand for effective gene therapies have spurred substantial investment in AAV vector development by numerous biotechnology companies.
Recent years have witnessed remarkable progress in AAV-based gene therapies, with 2023 marking a watershed moment for the field. The U.S. Food and Drug Administration (FDA) approved a record-breaking seven gene therapies that year, setting a new benchmark for the industry. This surge in approvals has catalyzed the growth of the global AAV therapy market, which reached $1.5 billion in 2023 and is projected to expand to $22.3 billion by 2029. Currently, six AAV-based gene therapies have received regulatory approval, with five gaining FDA authorization. The most recent approval in June 2023 underscores the rapid pace of innovation in this dynamic field.
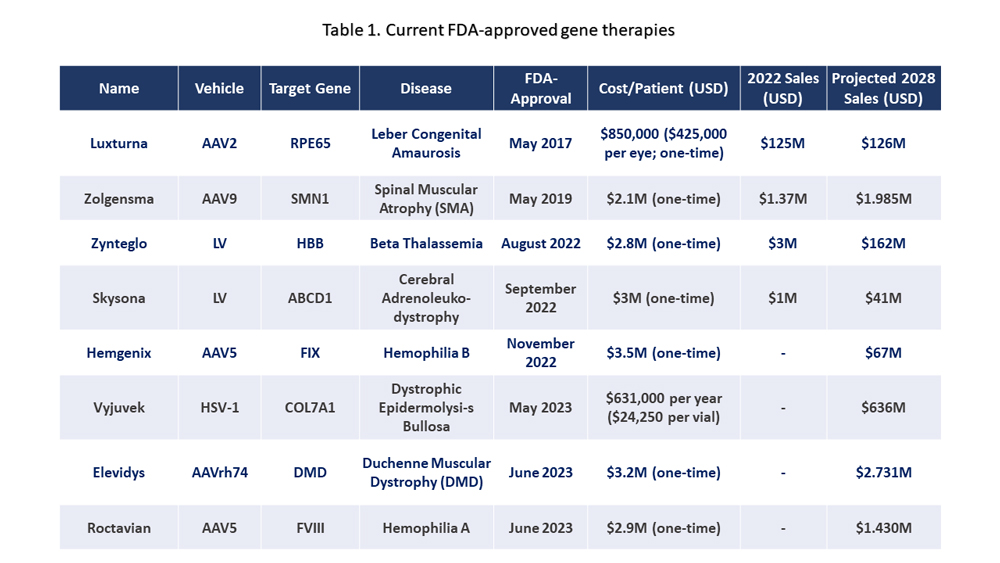
Table 1. Current FDA-approved gene therapies
As AAV vector applications in gene therapy continue to expand, the need for precise and reliable tools to support research and development becomes increasingly critical. Two fundamental aspects of AAV research are the accurate quantification of AAV capsid titers and the detection of pre-existing antibodies against AAV. These measurements are essential for ensuring the safety, efficacy, and proper dosing of gene therapies, as well as facilitating the successful translation of gene therapies from the laboratory to clinical applications.
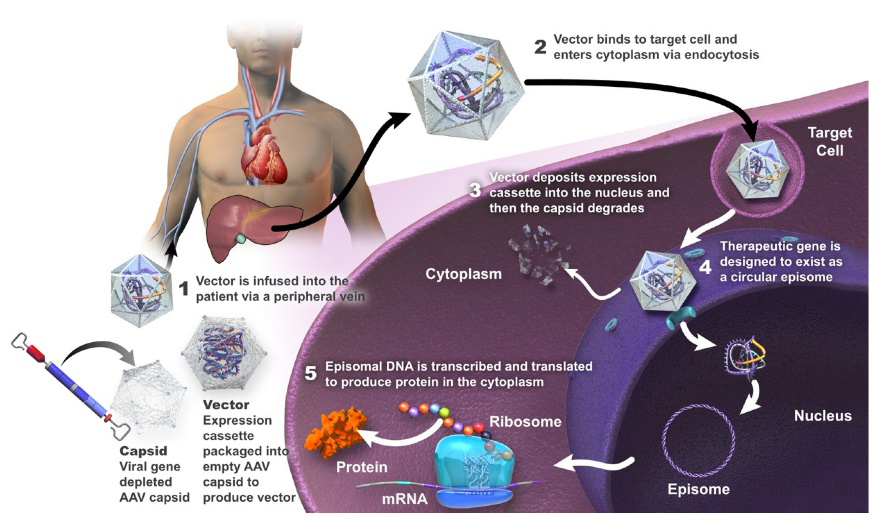
Figure 1. Summary of mechanism of action of gene therapy using AAV vector (adapted from https://encyclopedia.pub/entry/37119)
AAV therapy manufacturing involves complex processes where accurate capsid titration is crucial for quality control. Precise measurements ensure adequate viral capsid production, which is essential for therapeutic effectiveness in both preclinical and clinical phases. Consistent titer measurements guide dosing regimens, balancing efficacy and safety.
The AAV titration ELISA Kit provides precise quantification of AAV capsids in a biological sample, critical for determining correct therapeutic dosages and maintaining production QC. This kit ensures reliable measurements, optimizing production processes and meeting regulatory standards for clinical applications.
Regulatory bodies like the FDA require accurate viral titer measurements to meet identity, potency, quality, and purity standards. The AAV titration ELISA Kit can help support compliance with regulations by offering reproducible tools for measuring AAV capsid concentration. This facilitates regulatory submissions and approvals, advancing the development and commercialization of AAV-based therapies while potentially improving patient outcomes.
Pre-existing antibodies against AAV vectors present a significant challenge in AAV-mediated gene therapy, potentially compromising treatment efficacy in both human patients and animal models. While cell-based assays have traditionally been used to detect neutralizing antibodies, ELISA-based anti-AAV antibody detection methods have emerged as valuable and convenient tools for rapid initial screening.
ELISA-based anti-AAV antibody detection offers several advantages, including simpler operation, faster turnaround, and cost-effectiveness, especially when processing large sample numbers. A comparative study of AAV1, AAV8, and AAV9 serotypes in rhesus monkey and human sera revealed high consistency (Pearson r > 0.8) between ELISA and cell-based assays, validating ELISA as an effective screening method for both preclinical and clinical applications.
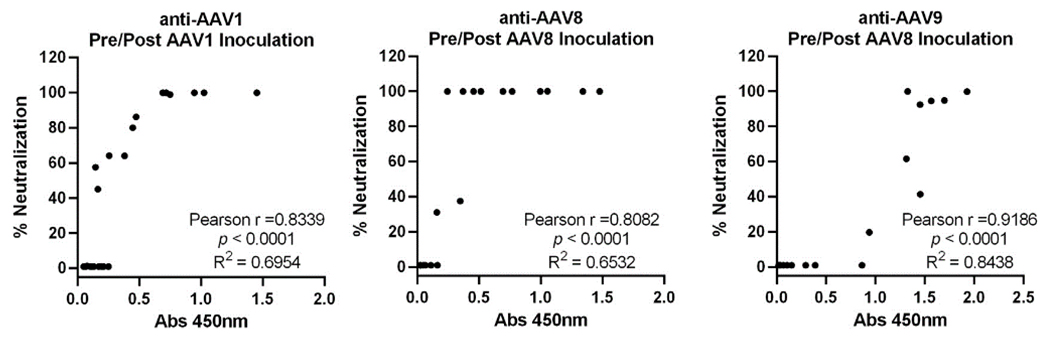
Figure 2. Correlation between ELISA and neutralization assays for rhesus macaque samples pre- and post-AAV inoculation Mol Ther Methods Clin Dev. 2022;24:199-206.
Anti-AAV antibody ELISA kits provide quantitative, reproducible results crucial for establishing antibody titer thresholds that may impact treatment outcomes. Their serotype-specific detection capabilities aid in selecting appropriate AAV vectors, while their ability to detect both neutralizing and non-neutralizing antibodies offers comprehensive immune response profiling. Although cell-based assays remain the gold standard for detailed characterization, ELISA kits offer a practical and efficient method for initial screening and monitoring.
AAV Titration and Anti-AAV Antibody ELISA kits serve as effective analytical tools in advancing AAV-based gene therapies. The AAV Titration ELISA Kit ensures precise quantification of viral capsids, facilitating accurate dosing and stringent quality control in vector production, while the Anti-AAV Antibody ELISA Kit enables efficient screening for pre-existing neutralizing antibodies that could impact treatment efficacy. Together, these assays enhance the robustness of gene therapy development by streamlining patient stratification, optimizing preclinical studies, and generating comprehensive data for regulatory submissions. As the field of AAV-based gene therapy continues to evolve, the integration of these standardized analytical methods not only enhances current research efficiency but also establishes a foundation for future innovations, ultimately accelerating the translation of promising therapeutic candidates from benchtop to clinic.
ACROBiosystems is pleased to announce the launch of a new series of AAV Titration ELISA kits and Anti-AAV Antibody ELISA kits, expertly designed to advance AAV gene therapy research. These kits feature exceptional sensitivity, specificity, and accuracy, enabling rapid and precise AAV titration and sample screening. Tailored for rigorous scientific applications, they provide researchers with reliable tools to advance their studies in gene therapy.
| AAV3 Titration ELISA Kit | AAV5 Titration ELISA Kit | AAV5 Titration ELISA Kit (Fast) |
| AAV8 Titration ELISA Kit | AAV8 Titration ELISA Kit (Fast) | Anti-AAV8 Antibody ELISA Kit |
| Anti-AAV9 Antibody ELISA Kit | AAV Capsid Titration Detection Antibody Pair (Universal) | … |
This web search service is supported by Google Inc.





