



























 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!
 Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!  Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!
> mAb for Anti-CD19 CAR Detection-Flow Cytometry Validated
CD19 is currently the most widely used target in CAR-T therapy, which has been validated to be effective and safe to treat B-ALL, CLL, and B cell lymphoma. FMC63 is an IgG2a mouse monoclonal antibody targeting CD19. So far, most of reported CART19 trials contain the anti-CD19 scFv derived from FMC63, including the two FDA-approved CARs Kymriah and Yescarta.
While the therapeutic outcomes for CD19-specific CAR-T cells have been impressive, the quality control during therapy manufacture remains an area in which more progress is required. Our high-affinity CD19 proteins can effectively detect the expression of anti-CD19 CAR on the surface of transduced T cells and have been widely recognized for their high sensitivity and specificity. These high-affinity CD19 proteins have been widely used for the quality control release testing and pharmacokinetic (PK) study of anti-CD19 CAR-T cells in clinical trials.
After receiving positive feedback on high-affinity CD19 proteins in market, ACROBiosystems now developed an anti-idiotypic antibody against FMC63 scFv, named Monoclonal Anti-FMC63 scFv Antibody, which can specifically recognize the antigen-recognition domain of FMC63 derived anti-CD19 CARs with high sensitivity and high specificity. The performance of anti-FMC63 scFv monoclonal antibody was validated by flow cytometry (FCM) in house and it is suitable for detecting FMC63 derived anti-CD19 CARs in clinical trials.
It is designed for cell isolation and cell culture applications, and produced under animal-free manufacturing conditions. No animal derived components are used throughout the production process. It is produced under our rigorous quality control system that incorporates a comprehensive set of tests including sterility and endotoxin tests. Product performance is carefully validated and tested for compatibility for cell culture use or any other applications in the early preclinical stage.
| Molecule | Cat. No. | Source | Product Description |
|---|
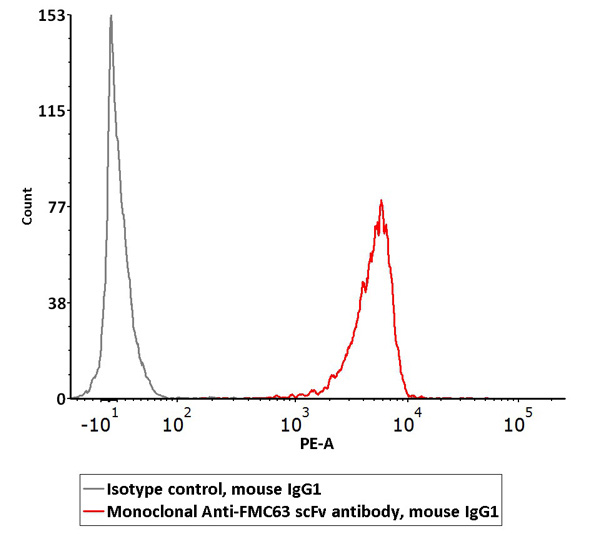
2e5 of FMC63 scFv-based anti-CD19 CAR-293 cells were stained with 100 µL of the working solution of Monoclonal AntiFMC63 scFv Antibody, Mouse IgG1 (Cat. No. FM3-Y45) and isotype control respectively, washed and then followed by PE anti-mouse IgG1 Antibody and analyzed with flow cytometry (QC tested).
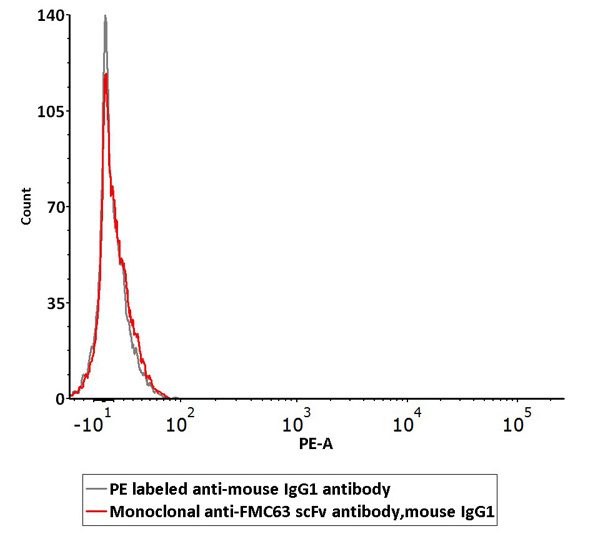
Non-specific binding of Monoclonal Anti-FMC63 scFv Antibody (Cat. No. FM3-Y45) to non-transfected 293 cells was determined by flow cytometry. The data showed that Anti-FMC63 scFv Antibody didn’t bind to non-transfected 293 cells.
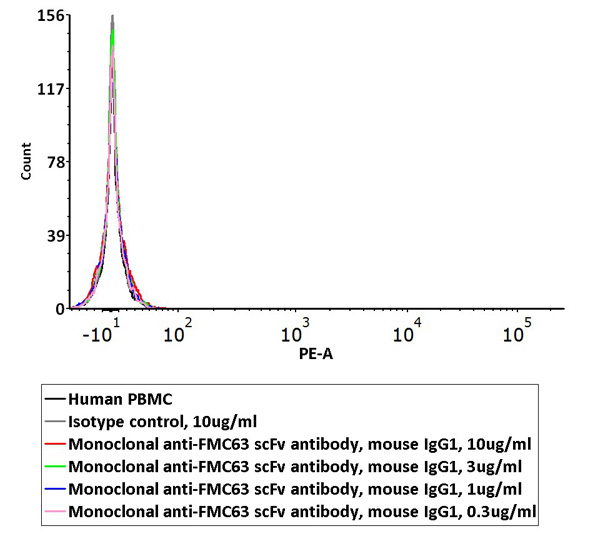
Non-specific binding of Monoclonal Anti-FMC63 scFv Antibody (Cat. No. FM3-Y45) to non-transfected human PBMCs was determined by flow cytometry. The data showed that Anti-FMC63 scFv Antibody didn’t bind to non-transfected human PBMCs.
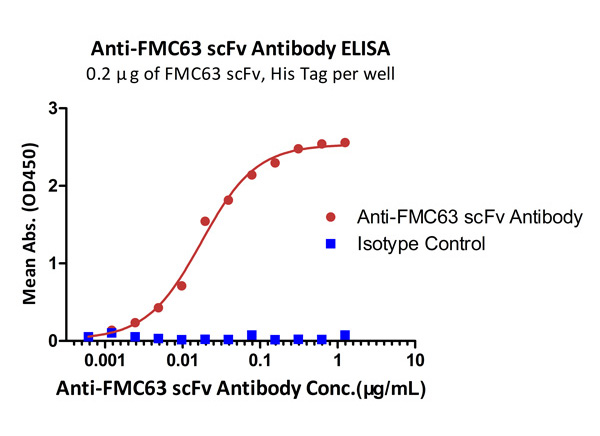
Immobilized FMC63 scFv, His Tag at 2 μg/mL (100 μL/well) can bind Monoclonal Anti-FMC63 scFv Antibody, Mouse IgG1 (Clone Y45) with a linear range of 1-19 ng/mL. Anti-DNP antibody, mouse IgG1 (Cat. No. DNP-M1) was used as an isotype control (QC tested).
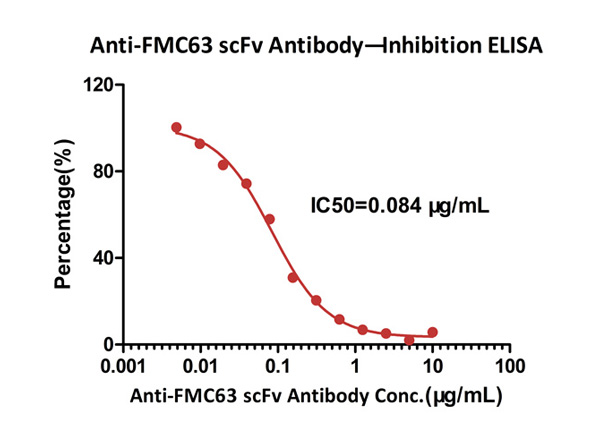
ELISA analysis shows that the binding of Human CD19, Fc Tag (Cat. No. CD9-H5251) to FMC63 scFv, His Tag was inhibited by increasing concentration of Monoclonal Anti-FMC63 scFv Antibody, Mouse IgG1 (Clone Y45). The concentration of Human CD19, Fc Tag used is 5 μg/mL (100 μL/well). The IC50 is 0.084 μg/mL (Routinely tested).
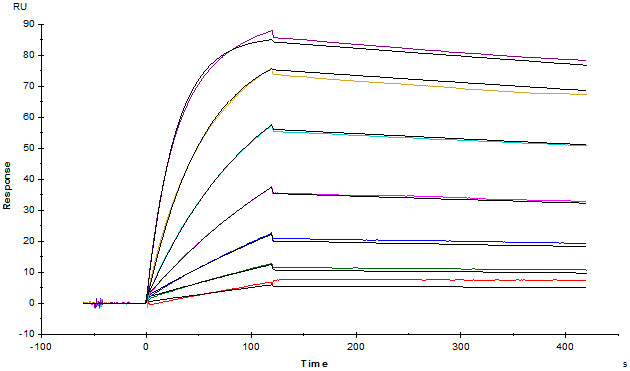
Monoclonal Anti-FMC63 scFv Antibody, Mouse IgG1 (Cat. No. FM3-Y45) captured on CM5 chip via anti-mouse antibodies surface can bind FMC63 scFv with an affinity constant of 1.08 nM as determined in a SPR assay.
| Molecule | Cat. No. | Source | Product Description | Structure |
|---|
This web search service is supported by Google Inc.









