



























 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!
 Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!  Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!
> Insights > Isolated antibodies in SARS-CoV-2 infected patients have potential to treat COVID-19 The pandemic caused by emerging coronavirus SARS-CoV-2 presents a serious global public health emergency in urgent need of prophylactic and therapeutic interventions. Scientists working to quell the COVID-19 pandemic think it will be possible to isolate monoclonal antibodies (mAbs) in SARS-CoV-2 infected patients and identify which antibodies are the most potent in quashing a coronavirus infection, and then make vast quantities of identical copies of these proteins synthetically to treat COVID-19.
Recently a team of Chinese scientists published a research paper1 on bioRxiv, the preprint server for Biology, reporting the isolation and characterization of mAbs derived from single B cells of eight SARS-CoV-2 infected individuals. The highlights of this study are summarized here:
1. Serial dilutions of plasma samples from eight SARS-CoV-2 infected subjects were analyzed for binding to RBDs from SARS-CoV-2, SARS-CoV and MERS-CoV. The plasma samples showed binding activity to SARS-CoV-2 RBD, while no cross-reactivity was detected against SARS-CoV RBD and MERS-CoV RBD. The results suggest that the RBD-based antibody response is viral species-specific (Fig 1).
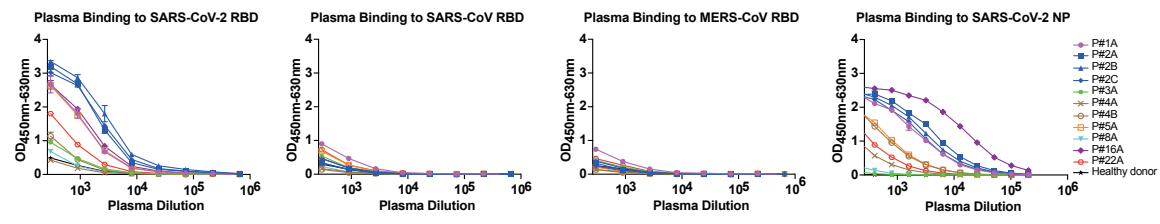
Fig 1. Analyses of plasma responses specific to SARS-CoV-2.
2. Flow cytometry was used to study SARS-CoV-2-specific B cell responses and identify B cells recognizing fluorescent-labeled RBD probes (Fig.2). They further isolated RBD-binding B cells into single cell suspension for cloning and obtained 206 mAbs that bound to SARS-CoV-2 RBD.
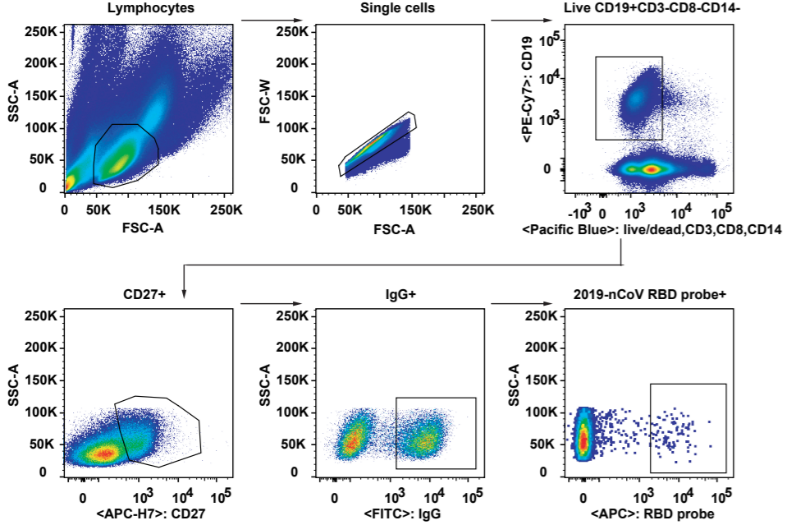
Fig 2. Gating strategy for analysis and isolation of SARS-CoV-2 RBD-specific memory B cells.
3. They selected 18 antibody clones based on their distribution on the phylogenetic tree and detected their binding to SARS-CoV-2 RBD using surface plasmon resonance (SPR). The dissociation constants of the antibodies ranged from 10-8 to 10-9 M, while none of the antibodies demonstrated cross-binding with SARS-CoV RBD and MERS-CoV RBD (Fig 3).
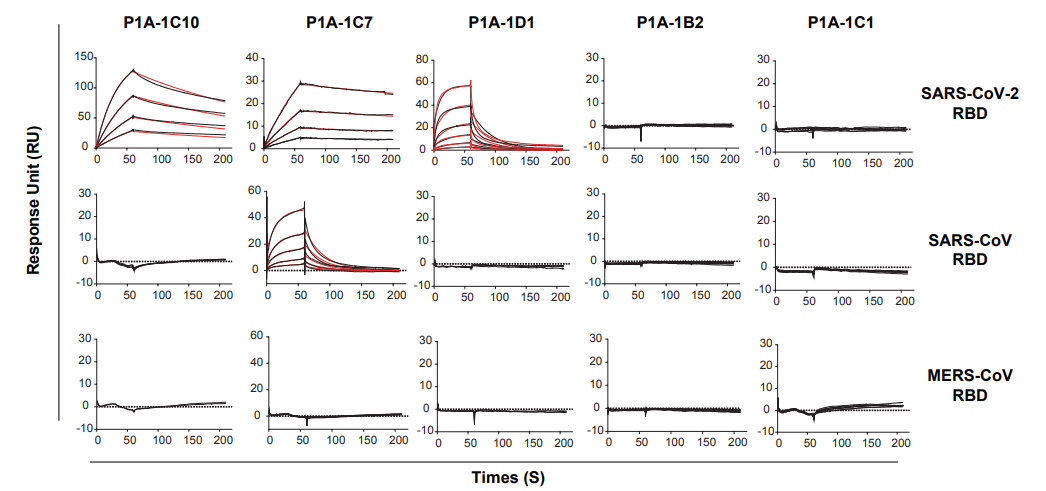
Fig 3. Binding kinetics of selected mAbs with SARS-CoV-2 RBD, SARS-CoV RBD and MERS-CoV RBD measured by SPR.
4. They measured the 18 antibodies for competition with ACE2 for binding to the SARS-CoV-2 RBD. While many antibodies showed strong binding to ACE2, they had only limited competing power with ACE2, suggesting binding affinity is not predictive of ACE2 competing capacity (Fig 4).
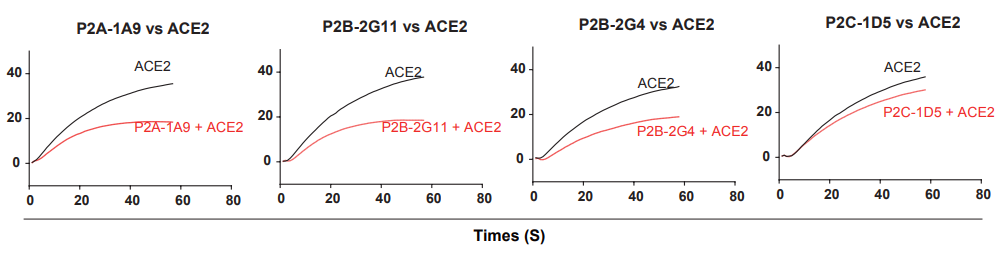
Fig 4. Antibody and ACE2 competition for binding to SARS-CoV-2 RBD measured by SPR.
5. They studied antibody neutralizing activities against pseudoviruses bearing the Spike protein of SARS-CoV-2. The neutralizing activities correlated well with the ACE2 competing capacity and in particular, the most potent antibodies (299 P2C-1F11 and P2B-2F6) out-competed ACE2 with close to 100% efficiency, indicating that blocking the RBD and ACE2 interaction is a useful surrogate for antibody neutralization (Fig 5).
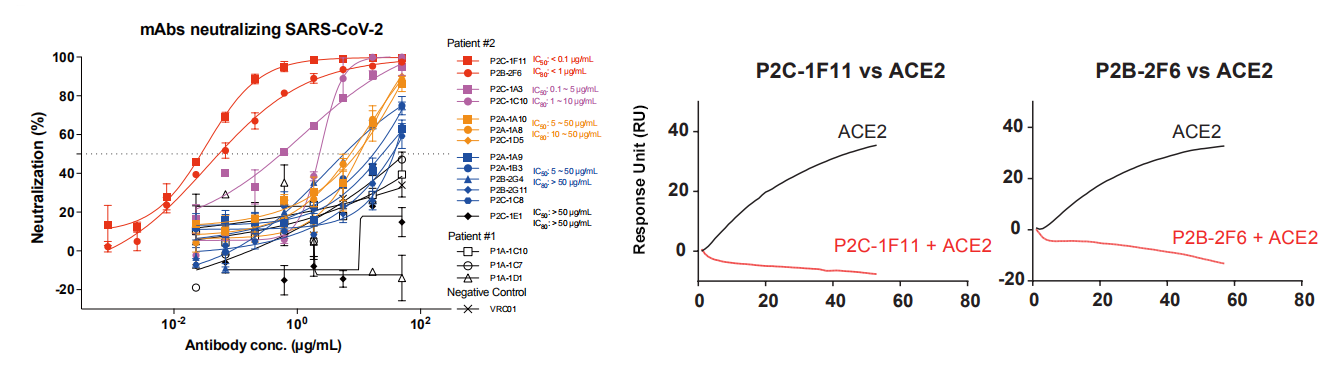
Fig 5 Antibody neutralization analyzed by pseudovirus.
The diverse and potent neutralizing antibodies identified in this study are promising candidates for prophylactic and therapeutic SARS-CoV-2 interventions. Currently the scientists are conducting pre-clinical studies in hoping to translate the identified mAbs into anti-COVID-19 drugs.
As the research paper suggested, blocking the RBD and ACE2 interaction is a useful surrogate for antibody neutralization. However, the low-throughput of SPR instruments limits the screening of large amounts of candidate drugs including neutralizing antibodies and small molecules. To solve this problem, ACROBiosystems develops ‘SARS-CoV-2 Inhibitor Screening Kit’, which employs colorimetric ELISA platform to measure the inhibitory effect of potential inhibitors on blocking the binding between immobilized SARS-CoV-2 S protein RBD and in-house developed biotinylated human ACE2 protein (Fig 6). This product would enable researchers to conduct high-throughput screening of SARS-CoV-2 inhibitors and speed up the development of therapeutics against COVID-19.
Reference (click below to download the PDF version of the literature ):
1. Potent human neutralizing antibodies elicited by SARS-CoV-2 infection.
This web search service is supported by Google Inc.





